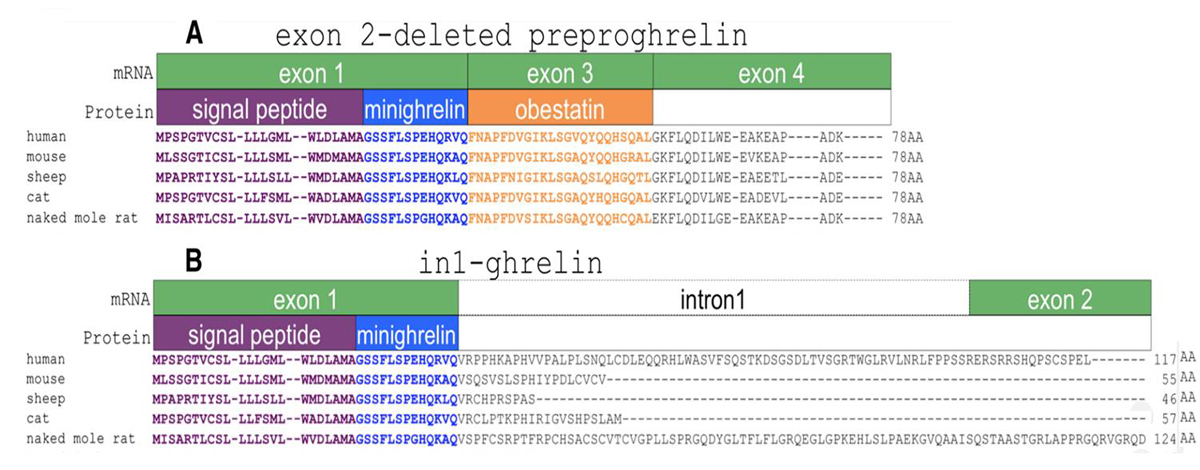
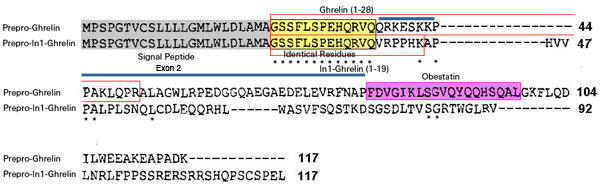
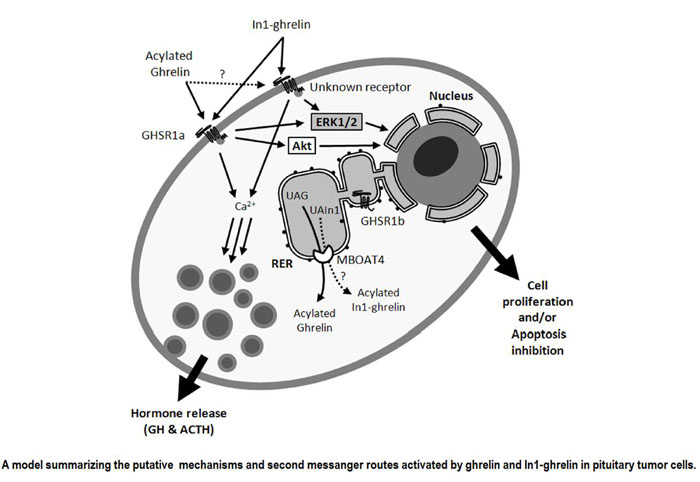
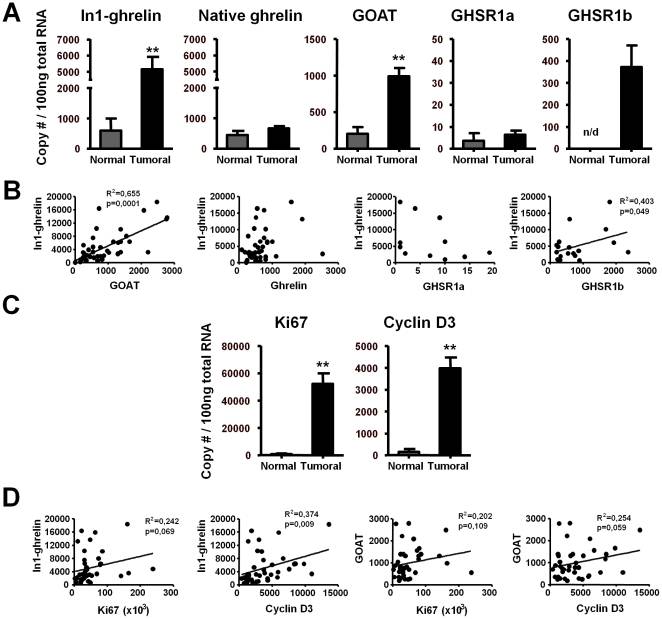
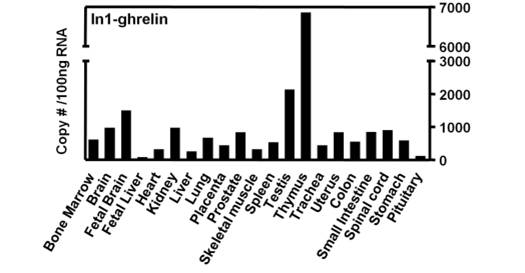
The human ghrelin gene, which encodes the ghrelin and obestatin peptides, contains 5 exons (Ex), with Ex1-Ex4 encoding a 117 amino-acid (aa) preproprotein that is known to be processed to yield a 28-aa (ghrelin) and/or a 23-aa (obestatin) mature peptides, which possess biological activities in multiple tissues. However, the ghrelin gene also encodes additional peptides through alternative splicing or post-translational modifications. Indeed, we previously identified a spliced mRNA ghrelin variant in mouse (In2-ghrelin-variant), which is regulated in a tissue-dependent manner by metabolic status and may thus be of biological relevance. Here, we have characterized a new human ghrelin variant that contains Ex0-1, intron (In) 1, and Ex2 and lacks Ex3-4. This human In1-ghrelin variant would encode a new prepropeptide that conserves the first 12aa of native-ghrelin (including the Ser3-potential octanoylation site) but has a different C-terminal tail. Expression of In1-variant was detected in 22 human tissues and its levels were positively correlated with those of ghrelin-O-acyltransferase (GOAT; p?=?0.0001) but not with native-ghrelin expression, suggesting that In1-ghrelin could be a primary substrate for GOAT in human tissues. Interestingly, levels of In1-ghrelin variant expression in breast cancer samples were 8-times higher than those of normal mammary tissue, and showed a strong correlation in breast tumors with GOAT (p=0.0001), ghrelin receptor-type 1b (GHSR1b; p=0.049) and cyclin-D3 (a cell-cycle inducer/proliferation marker; p=0.009), but not with native-ghrelin or GHSR1a expression. Interestingly, In1-ghrelin variant overexpression increased basal proliferation of MDA-MB-231 breast cancer cells. Taken together, our results provide evidence that In1-ghrelin is a novel element of the ghrelin family with a potential pathophysiological role in breast cancer.
Gahete et al., PLoS One. 2011;6(8):e23302. Epub 2011 Aug 4.
BACKGROUND: Peritoneal adhesion formation is a well-recognized consequence of abdominal and pelvic surgery, causing infertility, chronic pelvic pain, and intestinal obstruction. We hypothesized that ghrelin, a 28-amino acid peptide predominantly found in the stomach, plays an important role in preventing postoperative surgical adhesions. The purpose of this study was to develop a new surgical peritoneal adhesion model to define the role that ghrelin plays in wound healing and adhesion formation.
MATERIALS AND METHODS: C57BL/6 wild-type mice (n = 40) and growth hormone secretagogue receptor-knockout (GHSR KO) mice (n = 20) underwent a midline laparotomy to establish a peritoneal adhesion model characterized by the combination of two different techniques: ischemic peritoneal buttons and cecal multiple abrasion. All mice received intraperitoneal injections with ghrelin (0.16 mg/kg) or saline twice daily for 20 d after surgery. Peritoneal ischemic buttons were harvested to determine protein expression of collagen (Masson trichrome, picrosirius red stain, and Western blot).
RESULTS: The novel mouse model demonstrated consistent and easily reproducible formation of intra-abdominal adhesions. Ghrelin administration significantly reduced postoperative adhesion formation (P < 0.001) in wild-type mice. The antifibrotic effect of ghrelin in wild-type mice was confirmed by measuring collagen I protein levels via Western blot analysis. The anti-adhesion effect of ghrelin seen in wild-type mice was not detected in GHSR KO mice demonstrating that this effect is mediated by the GHSR-1a receptor.
CONCLUSIONS: Ghrelin administration may improve surgical outcome by reducing peritoneal adhesion formation and fibrotic response in a mouse model.Bianchi E, Boekelheide K, Sigman M, Lamb DJ, Hall SJ, Hwang K. Ghrelin ameliorates adhesions in a postsurgical mouse model. J Surg Res. 2016;201(1):226-34.
The peptide hormone ghrelin is a potent orexigen produced predominantly in the stomach. It has a number of other biological actions, including roles in appetite stimulation, energy balance, the stimulation of growth hormone release and the regulation of cell proliferation. Recently, several ghrelingene splice variants have been described. Here, we attempted to identify conserved alternative splicing of the ghrelin gene by cross-species sequence comparisons. We identified a novel human exon 2-deleted variant and provide preliminary evidence that this splice variant and in1-ghrelinencode a C-terminally truncated form of the ghrelin peptide, termed minighrelin. These variants are expressed in humans and mice, demonstrating conservation of alternative splicing spanning 90 million years. Minighrelin appears to have similar actions to full-length ghrelin, as treatment with exogenous minighrelin peptide stimulates appetite and feeding in mice. Forced expression of the exon 2-deleted preproghrelin variant mirrors the effect of the canonical preproghrelin, stimulating cell proliferation and migration in the PC3 prostate cancer cell line. This is the first study to characterise an exon 2-deleted preproghrelin variant and to demonstrate sequence conservation of ghrelin gene-derived splice variants that encode a truncated ghrelin peptide. This adds further impetus for studies into the alternative splicing of the ghrelin gene and the function of novel ghrelin peptides in vertebrates.
Seim I, Jeffery PL, Thomas PB, et al. Multi-species sequence comparison reveals conservation of ghrelin gene-derived splice variants encoding a truncated ghrelin peptide. Endocrine. 2016;52(3):609-17.
In the recent decades, great progress has been made in the development of ghrelin receptor ligands. The discovery of the first in vitro only active peptide growth hormone secretagogue derived from Met-enkephalin was the foundation for later discoveries of the receptor and the endogenous ligand ghrelin. Since then, the scope of peptides, peptidomimetics, and small-molecules targeting the ghrelin receptor, GHS-R1a, has expanded dramatically. Numerous agonists have been tested in animals and several in humans, and a handful have progressed to clinical trials for indications such as growth hormone release, gastric emptying, and cachexia. However, with the exception of the approval of GHRP-2 for diagnostic purposes in Japan, none of the candidates have been successfully introduced into the market. More recently, the attention of researchers has been concentrated on developing antagonists and inverse agonists for pharmacological treatment of the ever-expanding obese and overweight population. In this review, we describe the development of GHS-R1a targeting agonists, antagonists, and inverse agonists. We focus on current and completed clinical trials and the therapeutic potential of currently available ligands.
Vodnik M, Štrukelj B, Lunder M. Ghrelin Receptor Ligands Reaching Clinical Trials: From Peptides to Peptidomimetics; from Agonists to Antagonists. Horm Metab Res. 2016;48(1):1-15.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 031-31 | Ghrelin (Rat, Mouse) | 100 µg | $128 |
| EK-031-30 | Ghrelin (Human) - EIA Kit, extraction-free | 96 wells | $570 |
| 031-49 | Ghrelin (1-11) (Rat, Mouse, Porcine) | 100 µg | $242 |
| 031-30 | Ghrelin (Human) | 100 µg | $128 |
| RK-031-30 | Ghrelin (Human) - RIA Kit | 125 tubes | $816 |
| 031-52 | Ghrelin (Porcine) | 100 µg | $242 |
| EK-031-31 | Ghrelin (Rat, Mouse) - EIA Kit, extraction-free | 96 wells | $570 |
| EK-031-50 | Ghrelin (Canine) - EIA Kit, extraction-free | 96 wells | $570 |
| H-031-31 | Ghrelin (Rat, Mouse) - Antibody | 100 µl | $571 |
| 031-33 | [Ser3(Des-Octanoyl)]-Ghrelin (Rat, Mouse) | 100 µg | $189 |
Social Network Confirmation