 |
 |
Chemotherapy-induced bone marrow damage is accompanied by acute nerve injury in the bone marrow (BM), resulting in sensory and autonomic neuropathy. Cisplatin, a popular chemotherapy drugs, induces the impairment of hematopoietic stem cells (HSCs) and bone marrow regeneration, leading to chronic bone marrow abnormalities. Previously, we reported the protective roles of neuropeptide Y (NPY) against cisplatin-induced bone marrow impairment. In this study, we identified novel peptides, generated from full-length NPY that rescued cisplatin-induced sensory neuropathy and HSC suppression by regulating cell survival in the BM microenvironment. One of these peptides, especially, showed a better protective property against these impairments compared to that seen in full-length NPY. Therefore, we suggest the NPY sequences most effective against the chemotherapy-induced bone marrow dysfunction that could be potentially useful as therapeutic agents for patients receiving chemotherapy.Park MH, Baek B, Jin HK, Bae JS. Novel peptides derived from neuropeptide Y prevent chemotherapy-induced bone marrow damage by regulating hematopoietic stem cell microenvironment. Anim Cells Syst (Seoul). 2018;22(5):281-288.
Huntington's disease (HD) is an inherited and fatal polyglutamine neurodegenerative disorder caused by an expansion of the CAG triplet repeat coding region within the HD gene. Progressive dysfunction and loss of striatal GABAergic medium spiny neurons (MSNs) may account for some of the characteristic symptoms in HD patients. Interestingly, in HD, MSNs expressing neuropeptide Y (NPY) are spared and their numbers is even up-regulated in HD patients. Consistent with this, we report here on increased immuno-linked NPY (IL-NPY) levels in human cerebrospinal fluid (hCSF) from HD patients (Control n = 10; early HD n = 9; mid HD n = 11). As this antibody-based detection of NPY may provide false positive differences as a result of the antibody-based detections of only fragments of NPY, the initial finding was validated by investigating the proteolytic stability of NPY in hCSF using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and selective inhibitors. A comparison between resulting NPY-fragments and detailed epitope analysis verified significant differences in IL-NPY1-36/3-36 and NPY1-30 levels between HD patients and control subjects with no significant differences between early vs mid HD cases. Ex vivo degradomics analysis demonstrated that NPY is initially degraded to NPY1-30 by cathepsin D in both HD patients and control subjects. Yet, NPY1-30 is then further differentially hydrolyzed by thimet oligopeptidase (TOP) in HD patients and by neprilysin (NEP) in control subjects. Furthermore, altered hCSF TOP-inhibitor Dynorphin A1-13 (Dyn-A1-13 ) and TOP-substrate Dyn-A1-8 levels indicate an impaired Dyn-A-TOP network in HD patients. Thus, we conclude that elevated IL-NPY-levels in conjunction with TOP-/NEP-activity/protein as well as Dyn-A1-13 -peptide levels may serve as a potential biomarker in human CSF of HD. Huntington's disease (HD) patients' cerebrospinal fluid (CSF) exhibits higher neuropeptide Y (NPY) levels. Further degradomics studies show that CSF-NPY is initially degraded to NPY1-30 by Cathepsin D. The NPY1-30 fragment is then differentially degraded in HD vs control involving Neprilysin (NEP), Thimet Oligopeptidase (TOP), and TOP-Dynorphin-A network. Together, these findings may help in search for HD biomarkers.Wagner L, Björkqvist M, Lundh SH, et al. Neuropeptide Y (NPY) in cerebrospinal fluid from patients with Huntington's Disease: increased NPY levels and differential degradation of the NPY1-30 fragment. J Neurochem. 2016;137(5):820-37.
The endopeptidase neprilysin (NEP) is a major amyloid-beta (Abeta) degrading enzyme and has been implicated in the pathogenesis of Alzheimer's disease. Because NEP cleaves substrates other than Abeta, we investigated the potential role of NEP-mediated processing of neuropeptides in the mechanisms of neuroprotection in vivo. Overexpression of NEP at low levels in transgenic (tg) mice affected primarily the levels of neuropeptide Y (NPY) compared with other neuropeptides. Ex vivo and in vivo studies in tg mice and in mice that received lentiviral vector injections showed that NEP cleaved NPY into C-terminal fragments (CTFs), whereas silencing NEP reduced NPY processing. Immunoblot and mass spectrometry analysis showed that NPY 21-36 and 31-36 were the most abundant fragments generated by NEP activity in vivo. Infusion of these NPY CTFs into the brains of APP (amyloid precursor protein) tg mice ameliorated the neurodegenerative pathology in this model. Moreover, the amidated NPY CTFs protected human neuronal cultures from the neurotoxic effects of Abeta. This study supports the possibility that the NPY CTFs generated during NEP-mediated proteolysis might exert neuroprotective effects in vivo. This function of NEP represents a unique example of a proteolytic enzyme with dual action, namely, degradation of Abeta as well as processing of NPY.Rose JB, Crews L, Rockenstein E, et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J Neurosci. 2009;29(4):1115-25.
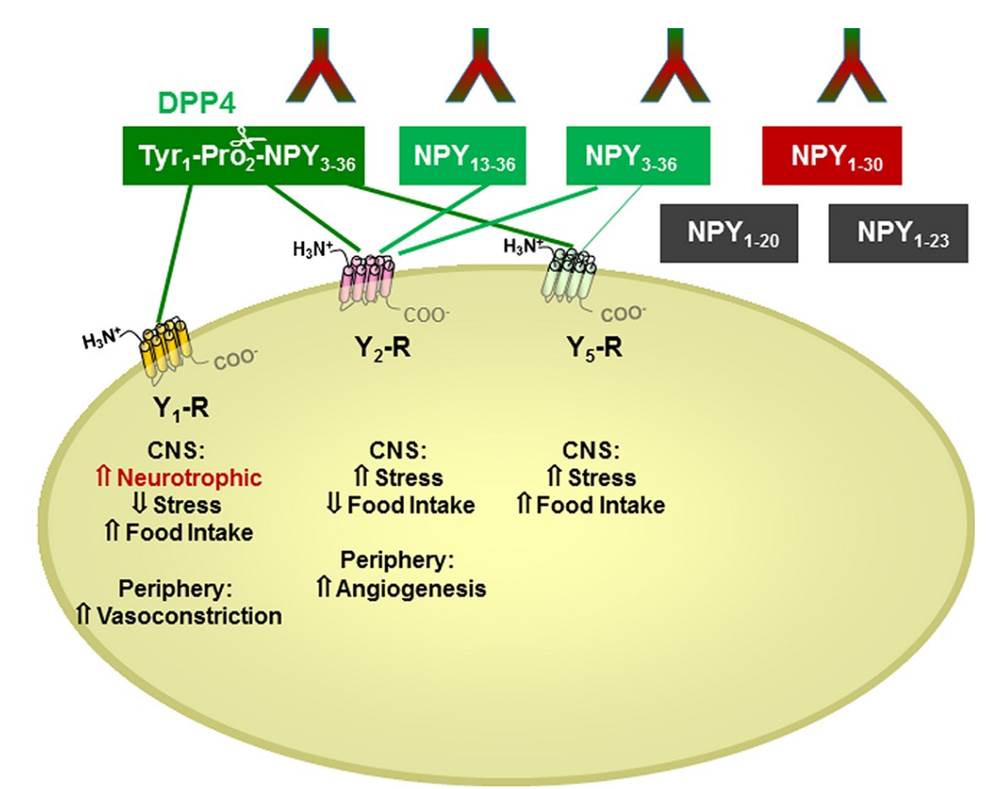
There is little information on how neuropeptide Y (NPY) proteolysis by peptidases occurs in serum, in part because reliable techniques are lacking to distinguish different NPY immunoreactive forms and also because the factors affecting the expression of these enzymes have been poorly studied. In the present study, LC-MS/MS was used to identify and quantify NPY fragments resulting from peptidolytic cleavage of NPY1–36 upon incubation with human serum. Kinetic studies indicated that NPY1–36 is rapidly cleaved in serum into 3 main fragments with the following order of efficacy: NPY3–36 ? NPY3–35 > NPY2–36. Trace amounts of additional NPY forms were identified by accurate mass spectrometry. Specific inhibitors of dipeptidyl peptidase IV, kallikrein, and aminopeptidase P prevented the production of NPY3–36, NPY3–35, and NPY2–36, respectively. Plasma kallikrein at physiological concentrations converted NPY3–36 into NPY3–35. Receptor binding assays revealed that NPY3–35 is unable to bind to NPY Y1, Y2, and Y5 receptors; thus NPY3–35 may represent the major metabolic clearance product of the Y2/Y5 agonist, NPY3–36.
Abid K, Rochat B, Lassahn PG, et al. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35: a new peptide generated by plasma kallikrein. J Biol Chem. 2009;284(37):24715-24
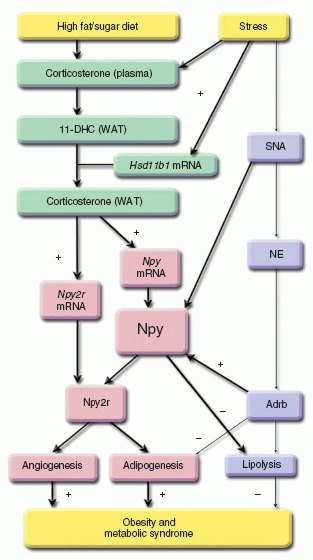
The relationship between stress and obesity remains elusive. In response to stress, some people lose weight, whereas others gain. Here we report that stress exaggerates diet-induced obesity through a peripheral mechanism in the abdominal white adipose tissue that is mediated by neuropeptide Y (NPY). Stressors such as exposure to cold or aggression lead to the release of NPY from sympathetic nerves, which in turn upregulates NPY and its Y2 receptors (NPY2R) in a glucocorticoid-dependent manner in the abdominal fat. This positive feedback response by NPY leads to the growth of abdominal fat. Release of NPY and activation of NPY2R stimulates fat angiogenesis, macrophage infiltration, and the proliferation and differentiation of new adipocytes, resulting in abdominal obesity and a metabolic syndrome-like condition. NPY, like stress, stimulates mouse and human fat growth, whereas pharmacological inhibition or fat-targeted knockdown of NPY2R is anti-angiogenic and anti-adipogenic, while reducing abdominal obesity and metabolic abnormalities. Thus, manipulations of NPY2R activity within fat tissue offer new ways to remodel fat and treat obesity and metabolic syndrome.
Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803-11
The first Y(5) receptor-selective analog of neuropeptide Y (NPY), [Ala(31),Aib(32)]NPY, has been developed and biologically characterized. Using competition binding assays on cell lines that express different Y receptors, we determined the affinity of this analog to be 6 nm at the human Y(5) receptor, >500 nm at the Y(1) and Y(2) receptors, and >1000 nm at the Y(4) receptor. Activity studies performed in vitro using a cAMP enzyme immunoassay, and in vivo using food intake studies in rats, showed that the peptide acted as an agonist. Further peptides obtained by the combination of the Ala(31)-Aib(32) motif with chimeric peptides containing segments of NPY and pancreatic polypeptide displayed the same selectivity and even higher affinity (up to 0.2 nm) for the Y(5) receptor. In vivo administration of the new Y(5) receptor-selective agonists significantly stimulated feeding in rats. The NMR solution structures of NPY and [Ala(31),Aib(32)]NPY showed a different conformation in the C-terminal region, where the alpha-helix of NPY was substituted by a more flexible, 3(10)-helical turn structure.
Cabrele C, Langer M, Bader R, et al. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem. 2000;275(46):36043-8
Immunoreactive-neuropeptide Y (i-NPY) is present in platelets of rats, and has recently been demonstrated to be authentic rat NPY based on its amino acid sequence. This potent vasoconstrictor and putative smooth muscle mitogen is released during platelet activation, suggesting a role in platelet-vascular interactions. We have now extended this work to several strains of rats and mice, and humans of both sexes. Among mice, strains in which NPY mRNA has been demonstrated in megakaryocytes have markedly higher levels of i-NPY (0.63-1.11 pmol/ml in NZB/B1NJ, NZBWF1/J, BXSB/MpJYaa, BALB/cJ) in platelet rich plasma (PRP) than other strains (DBA/2J, CBA/J, C3H/HeJ, MRL/MpJ-lpr, C57BL/6J; each < 0.02 pmol/ml). In rats, high content of i-NPY was observed in PRP and platelets of all strains examined (Sprague-Dawley, Wistar, Wistar Kyoto). i-NPY level was 30.6, 3.7 and 10.1 pmol/ml in PRP of the three strains, respectively. In humans, low levels of i-NPY occur in plasma and platelet fractions compared to rodents (0.069 and 0.048 pmol/ml in male and female PRP, respectively), but they, too, have greater i-NPY in platelet rich plasma and platelets than in platelet poor plasma. Assuming this is authentic NPY, platelet-derived NPY might have a role in pathophysiological states involving activation of platelets in humans.
Myers AK, Torres duarte AP, Zukowska-grojec Z. Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers. Regul Pept. 1993;47(3):239-4
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| T-049-08 | [Leu31, Pro34]-Neuropeptide Y (NPY) (Porcine) - I-125 Labeled | 10 µCi | $1082 |
| T-049-26 | [Ala31, Aib32]-Neuropeptide Y (NPY) (Porcine) - I-125 Labeled | 10 µCi | $1082 |
| T-G-049-07 | Neuropeptide Y (NPY) (Porcine) - I-125 Labeled Purified IgG | 10 µCi | $1082 |
| G-049-07 | Neuropeptide Y (NPY) (Porcine) - Purified IgG Antibody | 400 µg | $414 |
| RK-049-07 | Neuropeptide Y (NPY) (Porcine) - RIA Kit | 125 tubes | $880 |
| FR-049-07 | Neuropeptide Y (NPY) (Porcine) - Rhodamine Labeled | 1 nmol | $253 |
| 049-05 | Neuropeptide Y (NPY) free acid (Human, Rat, Mouse) | 200 µg | $97 |
Social Network Confirmation