Characterisation of G protein-coupled receptors (GPCR) relies on the availability of a toolbox of ligands that selectively modulate different functional states of the receptors. To uncover such molecules, we explored a unique strategy for ligand discovery that takes advantage of the evolutionary conservation of the 600-million-year-old oxytocin/vasopressin signalling system. We isolated the insect oxytocin/vasopressin orthologue inotocin from the black garden ant (Lasius niger), identified and cloned its cognate receptor and determined its pharmacological properties on the insect and human oxytocin/vasopressin receptors. Subsequently, we identified a functional dichotomy: inotocin activated the insect inotocin and the human vasopressin V1b receptors, but inhibited the human V1aR. Replacement of Arg8 of inotocin by D-Arg8 led to a potent, stable and competitive V1aR-antagonist ([D-Arg8]-inotocin) with a 3,000-fold binding selectivity for the human V1aR over the other three subtypes, OTR, V1bR and V2R. The Arg8/D-Arg8 ligand-pair was further investigated to gain novel insights into the oxytocin/vasopressin peptide-receptor interaction, which led to the identification of key residues of the receptors that are important for ligand functionality and selectivity. These observations could play an important role for development of oxytocin/vasopressin receptor modulators that would enable clear distinction of the physiological and pathological responses of the individual receptor subtypes.
Di giglio MG, Muttenthaler M, Harpsøe K, et al. Sci Rep. 2017;7:41002.
Ants have evolved venoms rich in peptides and proteins used for predation, defense, and communication. However, they remain extremely understudied due to the minimal amount of venom secreted by each ant. The present study investigated the differences in the proteome and peptidome of the venom from the bullet ant, Paraponera clavata. Venom samples were collected from a single colony either by manual venom gland dissection or by electrical stimulation and were compared using proteomic methods. Venom proteins were separated by 2D-PAGE and identified by nanoLC-ESI-QTOF MS/MS. Venom peptides were initially separated using C18 reversed-phase high-performance liquid chromatography, then analyzed by MALDI-TOF MS. The proteomic analysis revealed numerous proteins that could be assigned a biological function (total 94), mainly as toxins, or roles in cell regulation and transport. This investigation found that ca. 73% of the proteins were common to venoms collected by the two methods. The peptidomic analysis revealed a large number of peptides (total 309) but with <20% shared by the two collection methods. There was also a marked difference between venoms obtained by venom gland dissection from different ant colonies. These findings demonstrate the rich composition and variability of P. clavata venom.
Aili SR, Touchard A, Petitclerc F, et al. J Proteome Res. 2017;16(3):1339-1351.
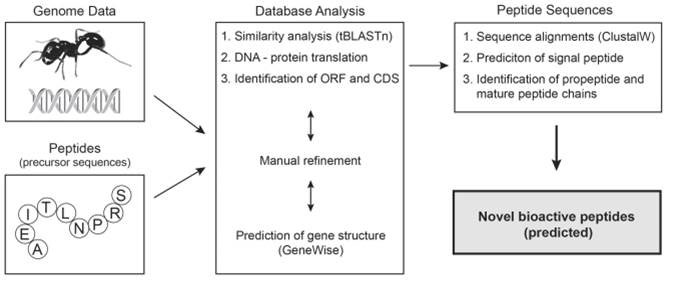
Natural peptides of great number and diversity occur in all organisms, but analyzing their peptidome is often difficult. With natural product drug discovery in mind, we devised a genome-mining approach to identify defense- and neuropeptides in the genomes of social ants from Atta cephalotes (leaf-cutter ant), Camponotus floridanus (carpenter ant) and Harpegnathos saltator (basal genus). Numerous peptide-encoding genes of defense peptides, in particular defensins, and neuropeptides or regulatory peptide hormones, such as allatostatins and tachykinins, were identified and analyzed. Most interestingly we annotated genes that encode oxytocin/vasopressin-related peptides (inotocins) and their putative receptors. This is the first piece of evidence for the existence of this nonapeptide hormone system in ants (Formicidae) and supports recent findings in Tribolium castaneum (red flour beetle) and Nasonia vitripennis (parasitoid wasp), and therefore its confinement to some basal holometabolous insects. By contrast, the absence of the inotocin hormone system in Apis mellifera (honeybee), another closely-related member of the eusocial Hymenoptera clade, establishes the basis for future studies on the molecular evolution and physiological function of oxytocin/vasopressin-related peptides (vasotocin nonapeptide family) and their receptors in social insects. Particularly the identification of ant inotocin and defensin peptide sequences will provide a basis for future pharmacological characterization in the quest for potent and selective lead compounds of therapeutic value.
Gruber CW, Muttenthaler M. PLoS ONE. 2012;7(3):e32559.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 065-51 | Inotocin | 100 µg | $181 |
| 065-53 | [D-Arg8]-Inotocin | 100 µg | $181 |
Social Network Confirmation