


The in vitro generation of uniform populations of neurons from mouse embryonic stem cells (ESCs) provides a novel opportunity to study gene function in neurons. This is of particular interest when mutations lead to lethal in vivo phenotypes. Although the amyloid precursor protein (APP) and its proteolysis are regarded as key elements of the pathology of Alzheimer's disease, the physiological function of APP is not well understood and mice lacking App and the related gene Aplp2 die early postnatally without any obvious histopathological abnormalities. Here we show that glutamatergic neurons differentiated from ESCs lacking both genes reveal a decreased expression of the vesicular glutamate transporter 2 (VGLUT2) both at the mRNA and protein level, as well as a reduced uptake and/or release of glutamate. Blocking gamma-secretase cleavage of APP in wild-type neurons resulted in a similar decrease of VGLUT2 expression, whereas VGLUT2 levels could be restored in App-/-Aplp2-/- neurons by a construct encompassing the C-terminal intracellular domain of APP. Electrophysiological recordings of hippocampal organotypic slice cultures prepared from corresponding mutant mice corroborated these observations. Gene expression profiling and pathway analysis of the differentiated App-/-Aplp2-/- neurons identified dysregulation of additional genes involved in synaptic transmission pathways. Our results indicate a significant functional role of APP and amyloid precursor-like protein 2 (APLP2) in the development of synaptic function by the regulation of glutamatergic neurotransmission. Differentiation of ESCs into homogeneous populations thus represents a new opportunity to explore gene function and to dissect signaling pathways in neurons.
Schrenk-Siemens K at el. Stem Cells. 2008 Aug;26(8):2153-63. Epub 2008 Jun 5.
The amyloid precursor protein (APP), the source of the neurotoxic amyloid beta (A beta) peptide involved in Alzheimer's disease (AD), belongs to a conserved family of related proteins. In mammals, the APP family contains amyloid precursor-like protein 1 (APLP1) and amyloid precursor-like protein 2 (APLP2). Whilst a number of activities have been attributed to the APP family, an overall function has not been definitively established. While ablating either the APP or APLP2 gene in mice produces minimal phenotypic change, the combined knockout of these genes in mice causes postnatal mortality. Postnatal survival therefore requires a shared but unknown function of APP and APLP2. To investigate the biochemical basis for the postnatal lethality, plasma was analysed from double knockout mice (APP-/- APLP2-/-) 2 days before birth, at gestational day E17, and from mice at 12-16 h after birth. The postnatal double knockouts had 66% lower plasma glucose levels than their wild-type controls and 50% lower than their single knockout counterparts. Interestingly, the postnatal double knockouts displayed hyperinsulinaemia, as shown by inappropriate plasma insulin levels, given their degree of hypoglycaemia. The single knockout mice also showed hyperinsulinaemia and had 31% lower plasma glucose than the wild-types. While the double knockouts did not survive more than 24 h after birth, the single knockouts reached adulthood and their hypoglycaemia continued. Therefore, APP and APLP2 expression modulates plasma insulin and glucose concentrations. Plasma calcium, magnesium and phosphate were also significantly reduced in the double knockouts compared to the wild-types, and they showed distinctive growth restriction, suggesting the involvement of a metabolic impairment. These results link the expression of the APP and APLP2 genes with glucose homeostasis and growth and therefore identify a novel function for the APP family.
Needham BE at el. J Pathol. 2008 Jun;215(2):155-63.
BACKGROUND Cerebrospinal fluid (CSF) levels of soluble amyloid precursor protein (sAPP) and its alpha-secreted form (alpha-sAPP) were investigated as a means to distinguish between individuals with mild cognitive impairment (MCI) and Alzheimer-type dementia (DAT) and those with major depressive episode (MDE) showing secondary memory deficits. METHODS Twenty-seven patients with MCI, 32 with probable DAT, and 24 with MDE attending a memory clinic were studied. Cerebrospinal fluid levels of sAPP/amyloid precursor-like protein 2 (APLP2) and alpha-sAPP were detected by Western blotting. RESULTS Patients with MDE had the highest CSF levels of total sAPP/APLP2 as compared with MCI and DAT patients (p < .001); sAPP/APLP2 levels were higher in MCI than in DAT subjects. Whereas alpha-sAPP levels did not differ between the MCI and DAT groups, median levels of this peptide were significantly lower in MCI and DAT versus MDE patients. CONCLUSIONS Soluble amyloid precursor protein/APLP2 and alpha-sAPP concentrations in CSF can differentiate between DAT and MCI versus MDE, facilitating early ameliorative interventions and appropriate treatment regimens.
Post A at el. Biol Psychiatry. 2006 May 1;59(9):858-62. Epub 2005 Dec 1
To facilitate clinical trials of disease-modifying therapies for Alzheimer’s disease, which are expected to be most efficacious at the earliest and mildest stages of the disease1,2, supportive biomarker information is necessary. The only validated methods for identifying amyloid-β deposition in the brain—the earliest pathological signature of Alzheimer’s disease—are amyloid-β positron-emission tomography (PET) imaging or measurement of amyloid-β in cerebrospinal fluid. Therefore, a minimally invasive, cost-effective blood-based biomarker is desirable3,4. Despite much effort3,4,5,6,7, to our knowledge, no study has validated the clinical utility of blood-based amyloid-β markers. Here we demonstrate the measurement of high-performance plasma amyloid-β biomarkers by immunoprecipitation coupled with mass spectrometry. The ability of amyloid-β precursor protein (APP)669–711/amyloid-β (Aβ)1–42 and Aβ1–40/Aβ1–42 ratios, and their composites, to predict individual brain amyloid-β-positive or -negative status was determined by amyloid-β-PET imaging and tested using two independent data sets: a discovery data set (Japan, n = 121) and a validation data set (Australia, n = 252 including 111 individuals diagnosed using 11C-labelled Pittsburgh compound-B (PIB)-PET and 141 using other ligands). Both data sets included cognitively normal individuals, individuals with mild cognitive impairment and individuals with Alzheimer’s disease. All test biomarkers showed high performance when predicting brain amyloid-β burden. In particular, the composite biomarker showed very high areas under the receiver operating characteristic curves (AUCs) in both data sets (discovery, 96.7%, n = 121 and validation, 94.1%, n = 111) with an accuracy approximately equal to 90% when using PIB-PET as a standard of truth. Furthermore, test biomarkers were correlated with amyloid-β-PET burden and levels of Aβ1–42 in cerebrospinal fluid. These results demonstrate the potential clinical utility of plasma biomarkers in predicting brain amyloid-β burden at an individual level. These plasma biomarkers also have cost–benefit and scalability advantages over current techniques, potentially enabling broader clinical access and efficient population screening.
Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249-254.
Regulated intramembrane proteolysis of the amyloid precursor protein (APP) and its homologs, the APP like proteins APLP1 and APLP2, is typically a two-step process, which is initiated by ectodomain-shedding of the substrates by α- or β-secretases. Growing evidence, however, indicates that the cleavage process for APLP1 is different than for APP. Here, we describe that full-length APLP1, but not APP or APLP2, is uniquely cleaved by γ-secretase without previous ectodomain shedding. The new fragment, termed sAPLP1γ, was exclusively associated with APLP1, not APP, APLP2. We provide an exact molecular analysis showing that sAPLP1γ was uniquely generated by γ-secretase from full-length APLP1. Mass spectrometry analysis showed that the sAPLP1γ fragment and the longest Aβ-like peptide share the C-terminus. This novel mechanism of γ-secretase action is consistent with an ε-cut based upon the nature of the reaction in APP. We further demonstrate that the APLP1 transmembrane sequence is the critical determinant for γ-shedding and release of full-length APLP1. Moreover, the APLP1 TMS is sufficient to convert larger type-I membrane proteins like APP into direct γ-secretase substrates. Taken together, the direct cleavage of APLP1 is a novel feature of the γ-secretase prompting a re-thinking of γ-secretase activity modulation as a therapeutic strategy for Alzheimer disease.
Schauenburg L, Liebsch F, Eravci M, Mayer MC, Weise C, Multhaup G. APLP1 is endoproteolytically cleaved by γ-secretase without previous ectodomain shedding. Sci Rep. 2018;8(1):1916.
BACKGROUND: Although APP and its proteolytic metabolites have been well examined in the central nervous system, there remains limited information of their functions outside of the brain. For example, amyloid precursor protein (APP) and amyloid beta (Aβ) immunoreactivity have both been demonstrated in intestinal epithelial cells. Based upon the critical role of these cells in absorption and secretion, we sought to determine whether APP or its metabolite amyloid β (Aβ), had a definable function in these cells.
METHODOLOGY/PRINCIPAL FINDINGS: The human colonic epithelial cell line, Caco-2 cells, were cultured to examine APP expression and Aβ secretion, uptake, and stimulation. Similar to human colonic epithelium stains, Caco-2 cells expressed APP. They also secreted Aβ 1-40 and Aβ 1-42, with LPS stimulating higher concentrations of Aβ 1-40 secretion. The cells also responded to Aβ 1-40 stimulation by increasing IL-6 cytokine secretion and decreasing cholesterol uptake. Conversely, stimulation with a sAPP-derived peptide increased cholesterol uptake. APP was associated with CD36 but not FATP4 in co-IP pull down experiments from the Caco-2 cells. Moreover, stimulation of APP with an agonist antibody acutely decreased CD36-mediated cholesterol uptake.
CONCLUSIONS/SIGNIFICANCE: APP exists as part of a multi-protein complex with CD36 in human colonic epithelial cells where its proteolytic fragments have complex, reciprocal roles in regulating cholesterol uptake. A biologically active peptide fragment from the N-terminal derived, sAPP, potentiated cholesterol uptake while the β secretase generated product, Aβ1-40, attenuated it. These data suggest that APP is important in regulating intestinal cholesterol uptake in a fashion dependent upon specific proteolytic pathways. Moreover, this biology may be applicable to cells beyond the gastrointestinal tract.
Puig KL, Manocha GD, Combs CK. Amyloid precursor protein mediated changes in intestinal epithelial phenotype in vitro. PLoS ONE. 2015;10(3):e0119534.
Alzheimer's disease (AD) is the most common and devastating dementia. Simple and practical biomarkers for AD are urgently required for accurate diagnosis and to facilitate the development of disease-modifying interventions. The subjects for the study were chosen on the basis of PiB amyloid imaging by PET. Forty PiB-positive (PiB+) individuals, including cognitively healthy controls (HC), and mild cognitive impairment and AD individuals, and 22 PiB-negative (PiB-) HC participated. Employing our novel highly sensitive immunoprecipitation-mass spectrometry, we measured plasma amyloid β-proteins (Aβs; Aβ1-40 and Aβ1-42) and Aβ-approximate peptides (AβAPs), which were cleaved from amyloid precursor protein (APP). Among the AβAPs, APP669-711 appeared to be a good reference for deciphering pathological change of Aβ1-42. We evaluated the performance of the ratio of APP669-711 to Aβ1-42 (APP669-711/Aβ1-42) as a biomarker. APP669-711/Aβ1-42 significantly increased in the PiB+ groups. The sensitivity and specificity to discriminate PiB+ individuals from PiB- individuals were 0.925 and 0.955, respectively. Our plasma biomarker precisely surrogates cerebral amyloid deposition.
Kaneko N, Nakamura A, Washimi Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad, Ser B, Phys Biol Sci. 2014;90(9):353-64.
Mutations in the amyloid precursor protein (APP) gene or in genes that process APP are correlated with familial Alzheimer’s disease (AD). The biological function of APP remains unclear. APP is a transmembrane protein that can be sequentially cleaved by different secretases to yield multiple fragments, which can potentially act as signaling molecules. Caenorhabditis elegans encodes one APP-related protein, APL-1, which is essential for viability. Here, we show that APL-1 signaling is dependent on the activity of the FOXO transcription factor DAF-16 and the nuclear hormone receptor DAF-12 and influences metabolic pathways such as developmental progression, body size, and egg-laying rate. Furthermore, apl-1(yn5) mutants, which produce high levels of the extracellular APL-1 fragment, show an incompletely penetrant temperature-sensitive embryonic lethality. In a genetic screen to isolate mutants in which the apl-1(yn5) lethality rate is modified, we identified a suppressor mutation in MOA-1/R155.2, a receptor-protein tyrosine phosphatase, and an enhancer mutation in MOA-2/B0495.6, a protein involved in receptor-mediated endocytosis. Knockdown of apl-1 in an apl-1(yn5) background caused lethality and molting defects at all larval stages, suggesting that apl-1 is required for each transitional molt. We suggest that signaling of the released APL-1 fragment modulates multiple metabolic states and that APL-1 is required throughout development.
Ewald C Y. Raps DA and Li C, Genetics. 2012 Jun;191(2):493-507. doi: 10.1534/genetics.112.138768. Epub 2012 Mar 30.
The relationship between altered metabolism of the amyloid-beta precursor protein (APP) and Alzheimer's disease is well established but the physiological roles of APP still remain unclear. Here, we studied Ca(2+) signaling in primary cultured and freshly dissociated cortical astrocytes from APP knockout (KO) mice and from Tg5469 mice overproducing by five- to six fold wild-type APP. Resting cytosolic Ca(2+) (measured with fura-2) was not altered in cultured astrocytes from APP KO mice. The stored Ca(2+) evaluated by measuring peak amplitude of cyclopiazonic acid [CPA, endoplasmic reticulum (ER) Ca(2+) ATPase inhibitor]-induced Ca(2+) transients in Ca(2+)-free medium was significantly smaller in APP KO astrocytes than in wild-type cells. Store-operated Ca(2+) entry (SOCE) activated by ER Ca(2+) store depletion with CPA was also greatly reduced in APP KO astrocytes. This reflected a downregulated expression in APP KO astrocytes of TRPC1 (C-type transient receptor potential) and Orai1 proteins, essential components of store-operated channels (SOCs). Indeed, silencer RNA (siRNA) knockdown of Orai1 protein expression in wild-type astrocytes significantly attenuated SOCE. SOCE was also essentially reduced in freshly dissociated APP KO astrocytes. Importantly, knockdown of APP with siRNA in cultured wild-type astrocytes markedly attenuated ATP- and CPA-induced ER Ca(2+) release and extracellular Ca(2+) influx. The latter correlated with downregulation of TRPC1. Overproduction of APP in Tg5469 mice did not alter, however, the stored Ca(2+) level, SOCE, and expression of TRPC1/4/5 in cultured astrocytes from these mice. The data demonstrate that the functional role of APP in astrocytes involves the regulation of TRPC1/Orai1-encoded SOCs critical for Ca(2+) signaling
Linde CI at el. Am J Physiol Cell Physiol. 2011 Jun;300(6):C1502-12. Epub 2011 Mar 2.
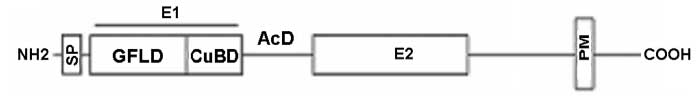
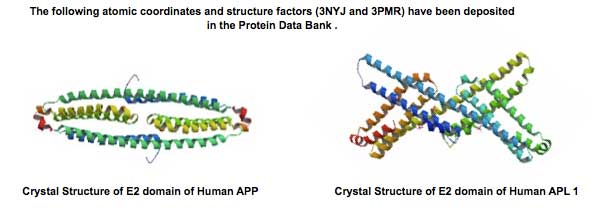
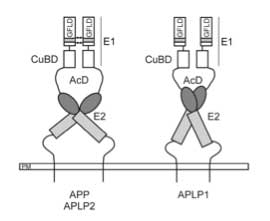
Amyloid precursor protein (APP) is genetically linked to Alzheimer's disease. APP is a type I membrane protein, and its oligomeric structure is potentially important because this property may play a role in its function or affect the processing of the precursor by the secretases to generate amyloid beta-peptide. Several independent studies have shown that APP can form dimers in the cell, but how it dimerizes remains controversial. At least three regions of the precursor, including a centrally located and conserved domain called E2, have been proposed to contribute to dimerization. Here we report two new crystal structures of E2, one from APP and the other from APLP1, a mammalian APP homologue. Comparison with an earlier APP structure, which was determined in a different space group, shows that the E2 domains share a conserved and antiparallel mode of dimerization. Biophysical measurements in solution show that heparin binding induces E2 dimerization. The 2.1 Å resolution electron density map also reveals phosphate ions that are bound to the protein surface. Mutational analysis shows that protein residues interacting with the phosphate ions are also involved in heparin binding. The locations of two of these residues, Arg-369 and His-433, at the dimeric interface suggest a mechanism for heparin-induced protein dimerization.
Lee S at el. Biochemistry. 2011 May 26.
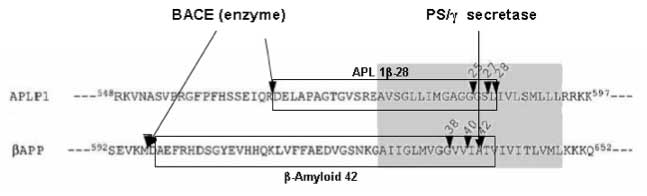
Alzheimer's disease (AD) is the most common cause of dementia in the elderly. Currently, therapeutic intervention after the disease onset is difficult because progressive neuronal death precedes clinical symptoms. Available medicines for AD, such as AchE inhibitors, transiently slow the progression of the dementia symptoms, but they do not inhibit the pathological process. At present, next generation anti-AD drugs are in development in many pharmaceutical companies. Importantly, most of them are to inhibit the progress of the pathological process and, thus, at the same time, the establishment of a highly probable prediction of future AD onset is inseparable. AD is now diagnosed using clinical criteria coupled with brain imaging systems such as SPECT and PET. To diagnose AD cases before the appearance of clinical symptoms, it will be necessary to (a) establish new, more sensitive clinical criteria, (b) develop methods for detecting the pathological accumulation of proteins (e.g. Abeta) in the brain, or (c) develop biomarkers for predicting the accumulation of Abeta/tau in the brain. Our recent discovery of APL1beta28, a possible biomarker of AD, may help in the development of early detection methods for AD
Okochi M at el. Neurodegener Dis. 2010;7(1-3):42-5. Epub 2010 Feb 13.
Chronic manganese (Mn) exposure produces a neurological syndrome with psychiatric, cognitive and parkinsonian features. Gene expression studies in the frontal cortex of Cynomolgus macaques exposed to different doses of Mn showed gene expression changes associated with cell cycle regulation, DNA repair, apoptosis, ubiquitin-proteasome system, protein folding, cholesterol homeostasis, axonal/vesicular transport and inflammation. Amyloid-beta (A-beta) precursor-like protein 1 (APLP1), a member of the amyloid precursor family, was the most highly up-regulated gene. Immunohistochemistry confirmed increased APLP1 expression and revealed the presence of A-beta diffuse plaques. Cortical neurons and white matter fibers from Mn-exposed animals exhibited accumulation of silver grains indicative of on-going degeneration. Cortical neurons also expressed nuclear hypertrophy, intracytoplasmic vacuoles, and apoptotis stigmata. The levels of p53 were increased in neurons and glial cells in Mn-exposed tissue. Analysis of another amyloidogenic protein, alpha-synuclein, also exhibited aggregation in the gray and white matter from Mn-exposed animals. In summary, chronic Mn exposure in non-human primates produces a cellular stress response leading to neurodegenerative changes, diffuse A-beta plaques and alpha-synuclein aggregation in the frontal cortex. These changes may help explain the cognitive and working memory deficits expressed by these animals.
Guilarte TR. Neurotoxicology. 2010 Sep;31(5):572-4. Epub 2010 Feb 25.
Surrogate markers for the Alzheimer disease (AD)-associated 42-amino acid form of amyloid-ß (Aß42) have been sought because they may aid in the diagnosis of AD and for clarification of disease pathogenesis. Here, we demonstrate that human cerebrospinal fluid (CSF) contains three APLP1-derived Aß-like peptides (APL1ß) that are generated by ß- and gamma-cleavages at a concentration of ~4.5 nM. These novel peptides, APL1ß25, APL1ß27 and APL1ß28, were not deposited in AD brains. Interestingly, most gamma-secretase modulators (GSMs) and familial AD associated presenilin1 mutants that up-regulate the relative production of Aß42 cause a parallel increase in the production of APL1ß28 in cultured cells. Moreover, in CSF from patients with pathological mutations in presenilin1 gene, the relative APL1ß28 levels are higher than in non-AD controls, while the relative Aß42 levels are unchanged or lower. Most strikingly, the relative APL1ß28 levels are higher in CSF from sporadic AD patients (regardless of whether they are at mild cognitive impairment or AD stage), than those of non-AD controls. Based on these results, we propose the relative level of APL1ß28 in the CSF as a candidate surrogate marker for the relative level of Aß42 production in the brain.Yanagida et. al. EMBO Molecular Medicine, Volume 1, Issue 4, Pages 223-235. Published Online: 11 Jun 2009
Ectodomain shedding of the amyloid precursor protein (APP) is a key regulatory step in the generation of the Alzheimer disease amyloid beta peptide (Abeta). The molecular mechanisms underlying the control of APP shedding remain little understood but are in part dependent on the low density lipoprotein receptor-related protein (LRP), which is involved in APP endocytosis. Here, we show that the APP homolog APLP1 (amyloid precursor-like protein 1) influences APP shedding. In human embryonic kidney 293 cells expression of APLP1 strongly activated APP shedding by alpha-secretase and slightly reduced beta-secretase cleavage. As revealed by domain deletion analysis, the increase in APP shedding required the NPTY amino acid motif within the cytoplasmic domain of APLP1. This motif is conserved in APP and is essential for the endocytosis of APP and APLP1. Unrelated membrane proteins containing similar endocytic motifs did not affect APP shedding, showing that the increase in APP shedding was specific to APLP1. In LRP-deficient cells APLP1 no longer induced APP shedding, suggesting that in wild-type cells APLP1 interferes with the LRP-dependent endocytosis of APP and there by increases APP alpha-cleavage. In fact, an antibody uptake assay revealed that expression of APLP1 reduced the rate of APP endocytosis. In summary, our study provides a novel mechanism for APP shedding, in which APLP1 affects the endocytosis of APP and makes more APP available for alpha-secretase cleavage.
Neumann S at el. J Biol Chem. 2006 Mar 17;281(11):7583-94. Epub 2005 Dec 12.
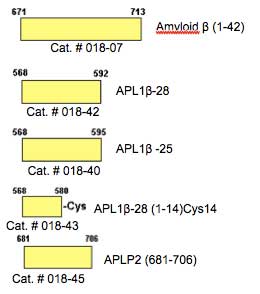
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 018-43 | [Cys14]-APL 1 beta 28 (1-14) | 100 µg | $189 |
| 018-40 | APL1 beta 25 | 100 µg | $230 |
| B-018-40 | APL1 beta 25 - Biotin Labeled | 20 µg | $382 |
| T-018-40 | APL1 beta 25 - I-125 Labeled | 10 µCi | $1082 |
| 018-41 | APL1 beta 27 | 100 µg | $242 |
| B-018-41 | APL1 beta 27 - Biotin Labeled | 20 µg | $382 |
| T-018-41 | APL1 beta 27 - I-125 Labeled | 10 µCi | $1082 |
| 018-42 | APL1 beta 28 | 100 µg | $253 |
| B-018-42 | APL1 beta 28 - Biotin Labeled | 20 µg | $382 |
| EK-018-42 | APL1 beta 28 - EIA Kit | 96 wells | $570 |
Social Network Confirmation