
|
|
Schematic representation of the solution structures of mBD-7 (A), mBD-8 (B) and hBD-1(C). The elements of secondary structure and the disulfide bonds are indicated. Same orientation as in Fig. 3a. The figure was drawn with Molscript (Kraulis 1991) and Raster 3D (Merritt and Murphy 1994). (D) Overlay of the structures of the two human defensins hBD-1 and hBD-2 (dark gray) with the structures of the two murine defensins mBD-7 and mBD-8 (light gray); figure drawn with SYBYL 6.5. BAUER F., et al. Protein Science (2001), 10:2470?479 |
|
|
Electrostatic surface plots of the most representative NMR structures of the three human β-defensins. HBD1, HBD2, and HBD3 are diagramed from left to right in the figure. In the top half of the figure the C-terminus of each of the structures is positioned on the right, and the N-terminal helix is at the top, whereas in the bottom half of the figure the structures are rotated 180?and the C-termini are now on the left, whereas the N-terminal helical regions remain at the top. The basic regions of the protein are colored blue, whereas the red regions are acidic. This figure was generated with GRASP (62). Schibli D. J., et al. J. Biol. Chem., Vol. 277, Issue 10, 8279-8289, March 8, 2002 |
|
|
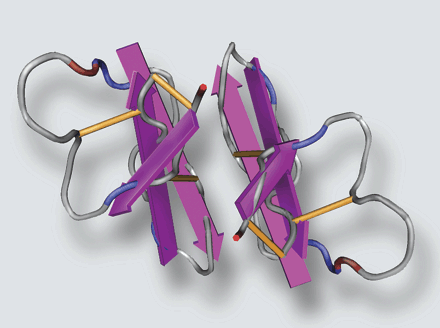
Possible orientations of two HBD3 monomers to form a dimer. Intermonomer hydrogen bonds between strand 2 of each of the monomers would establish a 6-stranded sheet. The dimer interface could be stabilized by the electrostatic interaction of Lys-32 and Glu-28 in addition to inter-monomer hydrogen bonds between the side chains of Gln-29. It should be noted that the positioning of the two monomers is only a model to illustrate the potential interactions if strand 2 were the dimer interface. Atoms of the two monomers may be overlapping, and correct bond distances have not been accounted for. This figure was created using the program MOLMOL. Schibli D. J., et al. J. Biol. Chem., Vol. 277, Issue 10, 8279-8289, March 8, 2002 |
|
|
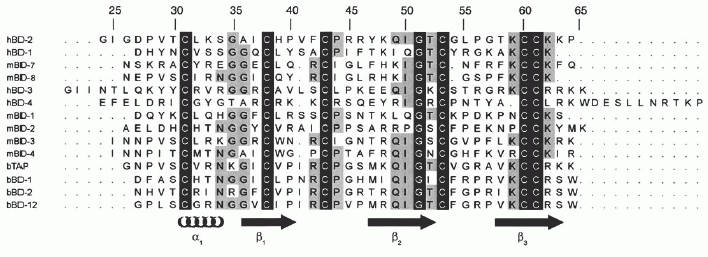
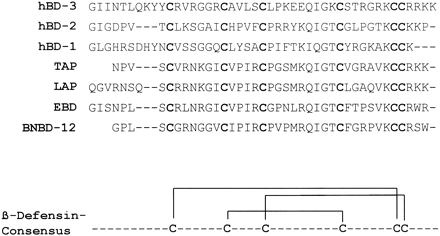
Sequence homology of HBD1-3 and bovine tracheal antimicrobial protein (TAP). Homologous residues among the defensins are highlighted. It should be noted that whereas the 42-residue sequence of HBD1 has been used for the alignment, the alignment does not alter for the 36-residue fragment used in this study. In addition to the sequence homology between the β-defensins, the net positive charge is also indicated. a, the net positive charge for both the 42-residue and the 36-residue HBD1 peptides is indicated. Alignment was performed with the ClustalX program . Schibli D. J., et al. J. Biol. Chem., Vol. 277, Issue 10, 8279-8289, March 8, 2002 |
|
|
Ribbon diagrams of the three human -defensins. A, HBD1; B, HBD2; C, HBD3. All three structures are in approximately the same orientation, with the three-disulfide bonds shown in gold. This figure was generated with the program MOLMOL. Schibli D. J., et al. J. Biol. Chem., Vol. 277, Issue 10, 8279-8289, March 8, 2002 |
|
|
Stereo-diagrams of the backbone trace of the 20 lowest energy structures of HBD3 with residues 6-44 overlaid (A) and HBD1 with residues 2-35 overlaid (B). The backbone and heavy atom r.m.s. distances of HBD3 are 0.616 and 1.337 for residues 6-45. The backbone and heavy atom r.m.s. distances of HBD1 are 0.451 and 0.992 for residues 2-35. This figure was generated with the program MOLMOL. |
AIM: To determine the concentration of alpja- and β-defensins in gastric juice of patients with various gastroduodenal diseases.
METHODS: Concentrations of human neutrophil peptides (HNPs) 1-3, the major forms of β-defensins, and human β-defensin (HBD)-1 and HBD-2 were measured by radioimmunoassay in plasma and gastric juice of 84 subjects, consisting of 54 Helicobacter pylori-infected and 30 uninfected subjects. They included 33 patients with chronic gastritis (CG), 12 with gastric ulcer (GU), 11 with duodenal ulcer (DU), 11 with benign gastric polyp (BGP) and 16 with normal mucosa (N group) on upper endoscopy. Plasma pepsinogen I and II levels, biomarkers for gastric mucosal inflammation and atrophy, were also measured.
RESULTS: Gastric juice HNPs 1-3 levels in patients with CG, GU and BGP were significantly higher than those in patients with DU and N. Gastric juice HBD-2 concentrations in patients with CG and GU were significantly higher than those in the N group, but were significantly lower in DU patients than in GU patients. Gastric juice HBD-1 levels and plasma levels of these peptides were similar in the patient groups. Concentrations of gastric juice HNPs 1-3 and HBD-2 of in H pylori-infected patients were significantly different from those in uninfected subjects. HNPs 1-3 concentrations in gastric juice correlated negatively with plasma pepsinogen I levels and I/II ratios. HBD-2 levels in gastric juice correlated positively and negatively with plasma pepsinogen II concentrations and I/II ratios, respectively.
CONCLUSION: HNPs 1-3 and HBD-2 levels in gastric juice are diverse among various gastrointestinal diseases, reflecting the inflammatory and atrophic events of the background gastric mucosa affected by H pylori.
Nishi Y, Isomoto H, Mukae H, et al. Concentrations of alpha- and beta-defensins in gastric juice of patients with various gastroduodenal diseases. World J Gastroenterol. 2005;11(1):99-103.
AIM: Human β-defensin (HBD)-1 and HBD-2 are endogenous antimicrobial peptides. Unlike HBD-1, the HBD-2 expression is augmented by Helicobacter pylori (H pylori). We sought to determine HBD-1 and HBD-2 concentrations in gastric juice during H pylori infection.
METHODS: HBD-1 and HBD-2 concentrations were measured by radioimmunoassay in plasma and gastric juice of 49 H pylori-infected and 33 uninfected subjects and before and after anti-H pylori treatment in 13 patients with H pylori-associated gastritis. Interleukin (IL)-1β and IL-8 concentrations in gastric juice were measured by enzyme-linked immunosorbent assay (ELISA). Histological grades of gastritis were determined using two biopsy specimens taken from the antrum and corpus. Reverse phase high performance liquid chromatography (RP-HPLC) was used to identify HBD-2. RESULTS: HBD-2 concentrations in gastric juice, but not in plasma, were significantly higher in H pylori-positive than -negative subjects, albeit the post-treatment levels were unchanged. Immunoreactivity for HBD-2 was exclusively identified in H pylori-infected mucosa by RP-HPLC. HBD-2 concentrations in gastric juice correlated with histological degree of neutrophil and mononuclear cell infiltration in the corpus. IL-1β levels correlated with those of IL-8, but not HBD-2. Plasma and gastric juice HBD-1 concentrations were similar in H pylori-infected and uninfected subjects.
CONCLUSION: Our results place the β-defensins, especially HBD-2, in the front line of innate immune defence. Moreover, HBD-2 may be involved in the pathogenesis of H pylori-associated gastritis, possibly through its function as immune and inflammatory mediator.
Isomoto H, Mukae H, Ishimoto H, et al. High concentrations of human β-defensin 2 in gastric juice of patients with Helicobacter pylori infection. World Journal of Gastroenterology?: WJG. 2005;11(31):4782-4787. doi:10.3748/wjg.v11.i31.4782.
ß-Defensins are important in mammalian immunity displaying both antimicrobial and chemoattractant activities. Three canonical disulfide intramolecular bonds are believed to be dispensable for antimicrobial activity but essential for chemoattractant ability. However, here we show that HBD3 (human β-defensin 3) alkylated with iodoactemide and devoid of any disulfide bonds is still a potent chemoattractant. Furthermore, when the canonical six cysteine residues are replaced with alanine, the peptide is no longer active as a chemoattractant. These findings are replicated by the murine ortholog Defb14. We restore the chemoattractant activity of Defb14 and HBD3 by introduction of a single cysteine in the fifth position (CysV) of the β-defensin six cysteine motif. In contrast, a peptide with a single cysteine at the first position (CysI) is inactive. Moreover, a range of overlapping linear fragments of Defb14 do not act as chemoattractants, suggesting that the chemotactic activity of this peptide is not dependent solely on an epitope surrounding CysV. Full-length peptides either with alkylated cysteine residues or with cysteine residues replaced with alanine are still strongly antimicrobial. Defb14 peptide fragments were also tested for antimicrobial activity, and peptides derived from the N-terminal region display potent antimicrobial activity. Thus, the chemoattractant and antimicrobial activities of β-defensins can be separated, and both of these functions are independent of intramolecular disulfide bonds. These findings are important for further understanding of the mechanism of action of defensins and for therapeutic design.
Taylor K, Clarke DJ, Mccullough B, et al. Analysis and separation of residues important for the chemoattractant and antimicrobial activities of beta-defensin 3. J Biol Chem. 2008;283(11):6631-9.
Infection of mucosal tissue and skin. We demonstrate that murine beta-defensin 2 (mDF2beta) acts directly on immature dendritic cells as an endogenous ligand for Toll-like receptor 4 (TLR-4), inducing up-regulation of costimulatory molecules and dendritic cell maturation. These events, in turn, trigger robust, type 1 polarized adaptive immune responses in vivo, suggesting that mDF2beta may play an important role in immunosurveillance against pathogens and, possibly, self antigens or tbeta-Defensins are small antimicrobial peptides of the innate immune system produced in response to microbial umor antigens.
Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025-9.
The growing public health problem of infections caused by multiresistant Gram-positive bacteria, in particularStaphylococcus aureus, prompted us to screen human epithelia for endogenous S. aureus-killing factors. A novel 5-kDa, nonhemolytic antimicrobial peptide (human β-defensin-3, hBD-3) was isolated from human lesional psoriatic scales and cloned from keratinocytes. hBD-3 demonstrated a salt-insensitive broad spectrum of potent antimicrobial activity against many potentially pathogenic microbes including multiresistant S. aureus and vancomycin-resistant Enterococcus faecium. Ultrastructural analyses of hBD-3-treated S. aureusrevealed signs of cell wall perforation. Recombinant hBD-3 (expressed as a His-Tag-fusion protein in Escherichia coli) and chemically synthesized hBD-3 were indistinguishable from naturally occurring peptide with respect to their antimicrobial activity and biochemical properties. Investigation of different tissues revealed skin and tonsils to be major hBD-3 mRNA-expressing tissues. Molecular cloning and biochemical analyses of antimicrobial peptides in cell culture supernatants revealed keratinocytes and airway epithelial cells as cellular sources of hBD-3. Tumor necrosis factor α and contact with bacteria were found to induce hBD-3 mRNA expression. hBD-3 therefore might be important in the innate epithelial defense of infections by various microorganisms seen in skin and lung, such as cystic fibrosis.Epithelia of macroorganisms represent the first barrier against invading microorganisms. However, despite constant exposure to these microbial threats, invasive infections and pathological disorders are rather rare and usually locally limited.Previous studies have demonstrated that plants and invertebrates produce a set of antimicrobial proteins that are highly effective at killing a wide variety of microorganisms (1). Although vertebrate epithelia are a rich source of antimicrobial proteins (2), it is a very recent observation that human epithelia mount an innate chemical defense by secreting antimicrobial peptides (3).The small (3–5 kDa) cationic defensins represent an important peptide family among antimicrobial peptides. Two subfamilies, the α-defensins and β-defensins, which are distinguished on the basis of the connectivity of their six cysteine residues, and more recently the cyclic θ-defensin from macaque leukocytes (4), have been identified in vertebrates (3). In humans two α-defensins, HD-5 and HD-6, are produced by epithelial granulocytes of the small intestine (5, 6).The first β-defensin was isolated from bovine tongue (7). Subsequently, 13 novel β-defensins were purified from bovine neutrophils (8), and the three-dimensional structure, including the disulfide array of one of these β-defensins, has been determined (9).The first isolated human β-defensin, human β-defensin-1 (hBD-1),1 was purified from hemofiltrates (10) and was later found in urine as a Gram-negative bacteria-killing antibiotic (11). mRNA of this antimicrobial peptide is constitutively expressed in various epithelia (10-14).The second human β-defensin, hBD-2, was discovered in extracts of lesional scales from patients suffering from psoriasis, a noninfectious proinflammatory and hyperproliferative skin disease (15, 16). hBD-2 is expressed in inflamed skin and lung and is induced in epithelial cells upon treatment with TNF-α (15, 17), interleukin-1β (17, 18), and contact with mucoid forms of Pseudomonas aeruginosa bacteria (17).Both human β-defensins show microbicidal activity predominantly against Gram-negative bacteria like Escherichia coli andP. aeruginosa. However, they demonstrate only low, if any, microbicidal activity against Gram-positive bacteria such asStaphylococcus aureus (3, 15, 19), a bacterium that causes infections ranging from skin abscesses to life-threatening conditions such as endocarditis and toxic shock (20).Recent investigations revealed that α-defensins also have the ability to attract T cells (21). Very recent investigations indicate that human β-defensins attract immature dendritic cells and memory T cells via the chemokine receptor CCR6 (22), providing a link between innate epithelial defense and adaptive immunity.Whereas skin infections caused by Gram-negative bacteria are rather rare, S. aureus is a major cause for skin and lung infections, in particular in atopic dermatitis (23). The high abundance of hBD-2 in skin (16) might explain its high resistance against Gram-negative bacterial infection. In contrast, the factors that protect skin from S. aureus infection remain speculative. We therefore hypothesized that human skin produces, in addition to the Gram-negative bacteria-killing hBD-2, peptide antibiotics directed against S. aureus. In the present study, we report the discovery of a novel human epithelial broad spectrum and multiresistant bacteria-killing peptide antibiotic, which we termed human β-defensin-3 (hBD-3) and which is inducibly expressed by various human epithelial cells.
Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276(8):5707-13.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-072-37 | Defensin 2, beta (Human) - ELISA Kit | 96 wells | $570 |
| 072-54 | Defensin 7, Beta (Mouse) | 100 µg | $415 |
| 072-42 | Defensin-3, Beta (Human) | 100 µg | $415 |
| 072-41A | Defensin-4 , Beta (Human) | 100 µg | $474 |
| 072-41B | Defensin-4, Beta (Human) | 20 µg | $237 |
| 072-48 | Defensin 2, beta, synthetic (Human) | 100 µg | $306 |
| EK-072-38 | Defensin 3, beta (Human) - ELISA Kit | 96 wells | $570 |
| 072-53 | Defensin 1, beta, synthetic (Human) | 20 µg | $202 |
| H-072-53 | Defensin 1, beta, synthetic (Human) - Antibody | 100 µl | $571 |
| B-G-072-53 | Defensin 1, beta, synthetic (Human) - Biotin Labeled Purified IgG | 100 µl | $629 |
Social Network Confirmation