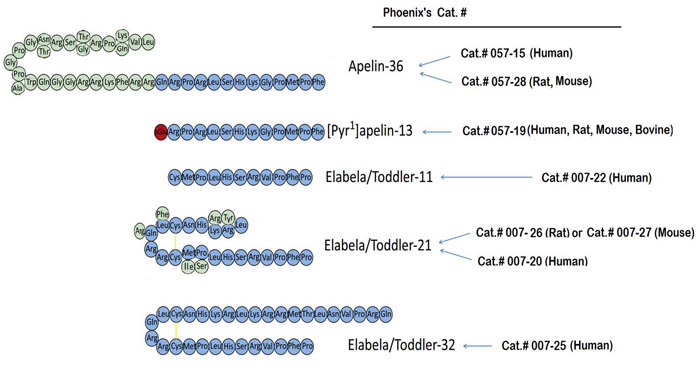
Sequences of apelin and Elabela/Toddler peptides, apelin-36, [Pyr1]apelin-13, Elabela/Toddler-32; Elabela/Toddler-21, Elabela/Toddler-11. Three-letter codes of amino acid residues are shown. pGlu, pyroglutamate; yellow lines represent disulfide bridges. Modified Figure from : Yang P el al. Trends Pharmacol Sci. 2015 Jul 1. pii: S0165-6147(15)00117-0. doi: 10.1016/j.tips.2015.06.002
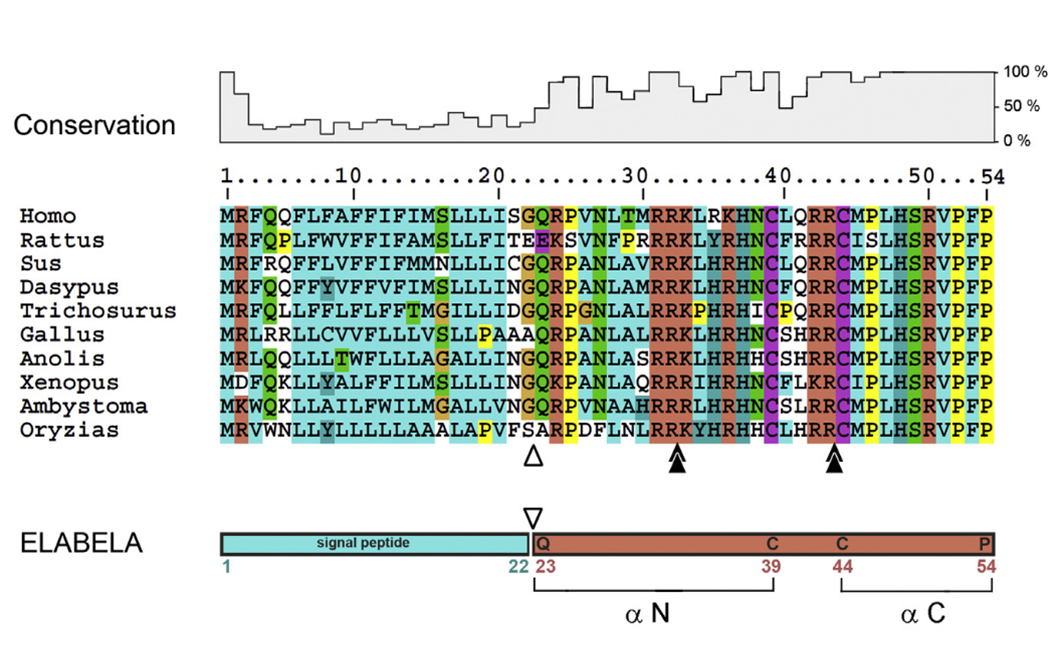
ELA encodes a conserved vertebrate protein of 54 aa consisting of a secretory signal and a mature 32 aa peptide. The carboxy terminus is invariant. White arrowhead: predicted signal peptide cleavage site between G22 and Q33. Black double arrowheads: possible furin cleavage sites after conserved di-arginines R31R32 and R42R43 motifs. The N- and C-terminal epitopes chosen for α N and α C antibody production are noted.
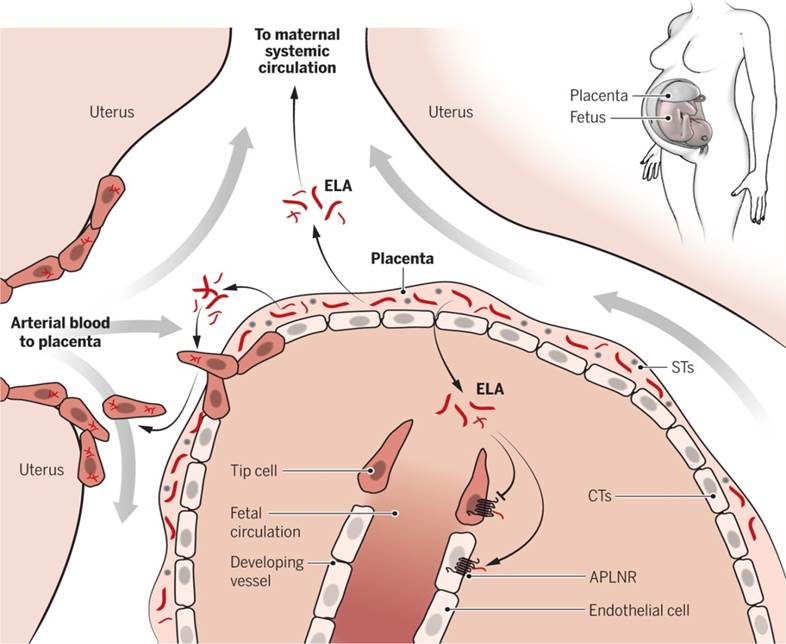
receptor (APLNR, or APJ), is a circulating hormone secreted by the placenta. Elabela but not Apelin knockout pregnant mice exhibit PE-like symptoms, including proteinuria and elevated blood pressure due to defective placental angiogenesis. In mice, infusion of exogenous ELA normalizes hypertension, proteinuria, and birth weight. ELA, which is abundant in human placentas, increases the invasiveness of trophoblast-like cells, suggesting that it enhances placental development to prevent PE. The ELA-APLNR signaling axis may offer a new paradigm for the treatment of common pregnancy-related complications, including PE.
Ho L, Van dijk M, Chye STJ, et al. Science. 2017;357(6352):707-713.
OBJECTIVES: Apelin-13 was recently proposed as an alternative to the recommended β-adrenergic drugs for supporting endotoxin-induced myocardial dysfunction. Since Apelin-13 signals through its receptor (Apelin peptide jejunum) to exert singular inotropic/vasotropic actions and to optimize body fluid balance, this candidate pathway might benefit septic shock management. Whether the newly discovered ELABELA(ELA), a second endogenous ligand of the Apelin peptide jejunum receptor highly expressed in the kidney, further improves cardio-renal impairment remains unknown.
DESIGN, SETTING, AND SUBJECTS: Interventional study in a rat model of septic shock (128 adult males) to assess the effects of ELA and Apelin-13 on vascular and cardio-renal function. Experiments were performed in a tertiary care University-based research institute.
INTERVENTIONS: Polymicrobial sepsis-induced cardiac dysfunction was produced by cecal ligation puncture to assess hemodynamic efficacy, cardioprotection, and biomechanics under acute or continuous infusions of the apelinergic agonists ELA or Apelin-13 (39 and 15 µg/kg/hr, respectively) versus normal saline.
MEASUREMENTS AND MAIN RESULTS: Apelinergic agonists improved 72-hour survival after sepsis induction, with ELA providing the best clinical outcome after 24 hours. Apelinergic agonist infusion counteracted cecal ligation puncture-induced myocardial dysfunction by improving left ventricular pressure-volume relationship. ELA-treated cecal ligation puncture rats were the only group to 1) display a significant improvement in left ventricular filling as shown by increased E-wave velocity and left ventricular end-diastolic volume, 2) exhibit a higher plasma volume, and 3) limit kidney injury and free-water clearance. These beneficial renal effects were superior to Apelin-13, likely because full-length ELA enabled a distinctive regulation of pituitary vasopressin release.
CONCLUSIONS: Activation of the apelinergic system by exogenous ELA or Apelin-13 infusion improves cardiovascular function and survival after cecal ligation puncture-induced sepsis. However, ELA proved better than Apelin-13 by improving fluid homeostasis, cardiovascular hemodynamics recovery, and limiting kidney dysfunction in a vasopressinergic-dependent manner.
Coquerel D, Chagnon F, Sainsily X, et al. Crit Care Med. 2017;
Renal ischemia-reperfusion (I/R) injury is the most common cause of AKI, which associates with high mortality and has no effective therapy. ELABELA (ELA) is a newly identified 32-residue hormone peptide highly expressed in adult kidney. To investigate whether ELA has protective effects on renal I/R injury, we administered the mature peptide (ELA32) or the 11-residue furin-cleaved fragment (ELA11) to hypoxia-reperfusion (H/R)-injured or adriamycin-treated renal tubular cells in vitro ELA32 and ELA11 significantly inhibited the elevation of the DNA damage response, apoptosis, and inflammation in H/R-injured renal tubular cells and suppressed adriamycin-induced DNA damage response. Similarly, overexpression of ELA32 or ELA11 significantly inhibited H/R-induced cell death, DNA damage response, and inflammation. Notably, treatment of mice with ELA32 or ELA11 but not an ELA11 mutant with a cysteine to alanine substitution at the N terminus (AE11C) inhibited I/R injury-induced renal fibrosis, inflammation, apoptosis, and the DNA damage response and markedly reduced the renal tubular lesions and renal dysfunction. Together, our results suggest that ELA32 and ELA11 may be therapeutic candidates for treating AKI.
Chen H, Wang L, Wang W et al., J Am Soc Nephrol. 2017 Jun 5. pii: ASN.2016111210. doi: 10.1681/ASN.2016111210.
Apela (also referred to as ELABELA and toddler) is a peptide hormone that activates the apelin receptor (AR or APJ) to regulate cardiovascular system development and function. Here, we report the first biophysical characterization of three apela isoforms, apela-54, -32, and -11, alongside a monomeric C1S-apela-11 mutant, using circular dichroism (CD) spectropolarimetry and nuclear magnetic resonance (NMR) spectroscopy. The behaviour of apela-54 is consistent with a preprotein containing a hydrophobic, N-terminal signal peptide. The potential for apela-membrane binding, leading to membrane catalyzed interactions with AR, was tested comprehensively for apela-32 and -11 in the presence of membrane-mimetic dodecylphosphocholine (DPC), sodium dodecyl sulfate (SDS), and 1-palmitoyl-2-hydroxy-sn-glycero-3-[phospho-rac-(1-glycerol)] (LPPG) micelles. According to pulsed-field gradient diffusion NMR experiments, apela-32 interacts with all three micelles. Chemical shift perturbations indicate widespread interactions along apela, with DPC and LPPG micelles inducing short segments with α-helical character at distinct regions. Consistent with these data, ps-ns dynamics along the peptide backbone appear decreased in the presence of micelles. Apela-11 and C1S-apela-11, alternatively, interact preferentially with SDS and LPPG micelles, promoting β-turn character observable by CD. Distinct differences in membrane-interaction propensity are therefore apparent both as a function of apela isoform and of detergent headgroup. These results imply the potential for cell membrane involvement in apela-AR recognition and binding, with the implication that membrane catalysis has distinct functional and regulatory roles throughout the apelinergic system.
Huang SK, Shin K, Sarker M, Rainey JK. Biochim Biophys Acta. 2017;1859(5):767-778.
BACKGROUND: Elabela/toddler (ELA) is a critical cardiac developmental peptide that acts through the G-protein-coupled apelin receptor, despite lack of sequence similarity to the established ligand apelin. Our aim was to investigate the receptor pharmacology, expression pattern, and in vivo function of ELA peptides in the adult cardiovascular system, to seek evidence for alteration in pulmonary arterial hypertension (PAH) in which apelin signaling is downregulated, and to demonstrate attenuation of PAH severity with exogenous administration of ELA in a rat model.
METHODS: In silico docking analysis, competition binding experiments, and downstream assays were used to characterize ELA receptor binding in human heart and signaling in cells expressing the apelin receptor. ELA expression in human cardiovascular tissues and plasma was determined using real-time quantitative polymerase chain reaction, dual-labeling immunofluorescent staining, and immunoassays. Acute cardiac effects of ELA-32 and [Pyr1]apelin-13 were assessed by MRI and cardiac catheterization in anesthetized rats. Cardiopulmonary human and rat tissues from PAH patients and monocrotaline- and Sugen/hypoxia-exposed rats were used to show changes in ELA expression in PAH. The effect of ELA treatment on cardiopulmonary remodeling in PAH was investigated in the monocrotaline rat model.
RESULTS: ELA competed for binding of apelin in human heart with overlap for the 2 peptides indicated by in silico modeling. ELA activated G-protein- and β-arrestin-dependent pathways. We detected ELA expression in human vascular endothelium and plasma. Comparable to apelin, ELA increased cardiac contractility, ejection fraction, and cardiac output and elicited vasodilatation in rat in vivo. ELA expression was reduced in cardiopulmonary tissues from PAH patients and PAH rat models, respectively. ELA treatment significantly attenuated elevation of right ventricular systolic pressure and right ventricular hypertrophy and pulmonary vascular remodeling in monocrotaline-exposed rats.
CONCLUSIONS: These results show that ELA is an endogenous agonist of the human apelin receptor, exhibits a cardiovascular profile comparable to apelin, and is downregulated in human disease and rodent PAH models, and exogenous peptide can reduce the severity of cardiopulmonary remodeling and function in PAH in rats. This study provides additional proof of principle that an apelin receptor agonist may be of therapeutic use in PAH in humans.
This publication used ELA peptide, antibody, and ELISA Kit from Phoenix Pharmaceuticals.
Yang P, Read C, Kuc RE, et al. Circulation. 2017;135(12):1160-1173.
Aims: Apelin is a predicted substrate for ACE2, a novel therapeutic target. Our aim was to demonstrate the endogenous presence of the putative ACE2 product [Pyr1]apelin-13(1-12) in human cardiovascular tissues and to confirm it retains significant biological activity for the apelin receptor in vitro and in vivo. The minimum active apelin fragment was also investigated.
Methods and Results: [Pyr1]apelin-13incubated with recombinant human ACE2 resulted in de novo generation of [Pyr1]apelin-13(1-12) identified by mass spectrometry. Endogenous [Pyr1]apelin-13(1-12) was detected by immunostaining in human heart and lung localized to the endothelium. Expression was undetectable in lung from patients with pulmonary arterial hypertension. In human heart [Pyr1]apelin-13(1-12) (pKi = 8.04 ± 0.06) and apelin-13(F13A) (pKi = 8.07 ± 0.24) competed with [125I]apelin-13 binding with nanomolar affinity, 4-fold lower than for [Pyr1]apelin-13 (pKi = 8.83 ± 0.06) whereas apelin-17 exhibited highest affinity (pKi = 9.63 ± 0.17). The rank order of potency of peptides to inhibit forskolin-stimulated cAMP was apelin-17 (pD2 = 10.31 ± 0.28) > [Pyr1]apelin-13 (pD2 = 9.67 ± 0.04) ? apelin-13(F13A) (pD2 = 9.54 ± 0.05) > [Pyr1]apelin-13(1-12)(pD2 = 9.30 ± 0.06). The truncated peptide apelin-13(R10M) retained nanomolar potency (pD2 = 8.70 ± 0.04) but shorter fragments exhibited low micromolar potency. In a ?-arrestin recruitment assay the rank order of potency was apelin-17 (pD2 = 10.26 ± 0.09) >> [Pyr1]apelin-13(pD2 = 8.43 ± 0.08) > apelin-13(R10M) (pD2 = 8.26 ± 0.17) > apelin-13(F13A) (pD2 = 7.98 ± 0.04) ? [Pyr1]apelin-13(1-12) (pD2 = 7.84 ± 0.06) >> shorter fragments (pD2 < 6). [Pyr1]apelin-13(1-12) and apelin-13(F13A) contracted human saphenous vein with similar sub-nanomolar potencies and [Pyr1]apelin-13(1-12) was a potent inotrope in paced mouse right ventricle and human atria. [Pyr1]apelin-13(1-12) elicited a dose-dependent decrease in blood pressure in anesthetized rat and dose-dependent increase in forearm blood flow in human volunteers.
Conclusions: We provide evidence that ACE2 cleaves [Pyr1]apelin-13 to [Pyr1]apelin-13(1-12) and this cleavage product is expressed in human cardiovascular tissues. We have demonstrated biological activity of [Pyr1]apelin-13(1-12) at the human and rodent apelin receptor in vitro and in vivo. Our data show that reported enhanced ACE2 activity in cardiovascular disease should not significantly compromise the beneficial effects of apelin based therapies for example in PAH.
This publication used ELA products from Phoenix Pharmaceuticals.
Chen H, Wang L, Wang W et al., J Am Soc Nephrol. 2017 Jun 5. pii: ASN.2016111210. doi: 10.1681/ASN.2016111210.
ELABELA (ELA) was recently discovered as a novel endogenous ligand of the apelin receptor (APJ), a G protein-coupled receptor. ELA signaling was demonstrated to be crucial for normal heart and vasculature development during embryogenesis. We delineate here ELA's structure-activity relationships and report the identification of analogue 3 (ELA(19-32)), a fragment of ELA that binds to APJ, activates the G?i1 and ?-arrestin-2 signaling pathways and induces receptor internalization similarly to its parent endogenous peptide. An alanine scan performed on 3 revealed that the C-terminal residues are critical for binding to APJ and signaling. Finally, using isolated-perfused hearts and in vivo hemodynamic and echocardiographic measurements, we demonstrate that ELA and 3 both reduce arterial pressure and exert positive inotropic effects on the heart. Altogether, these results present ELA and 3 as potential therapeutic options in managing cardiovascular diseases.
Murza A, Sainsily X, Coquerel D, et al. Discovery and Structure-Activity Relationship of a Bioactive Fragment of ELABELA that Modulates Vascular and Cardiac Functions. J Med Chem. 2016;59(7):2962-72.
The G protein-coupled apelin receptor regulates important processes of the cardiovascular homeostasis, including cardiac development, cardiac contractility, and vascular tone. Most recently, a novel endogenous peptide ligand for the apelin receptor was identified in zebrafish, and it was named apela/elabela/toddler. The peptide was originally considered as an exclusively embryonic regulator, and so far its function in the adult organism remains elusive. We show here that apela is predominantly expressed in the non-cardiomyocyte fraction in the adult rodent heart. We also provide evidence that apela binds to apelin receptors in the heart. Using isolated adult rat hearts, we demonstrate, that just like the fellow receptor agonist apelin, apela increases cardiac contractility and induces coronary vasodilation already in the nanomolar level. The inotropic effect, as revealed by Western blot analysis, is accompanied by a significant increase in extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. Pharmacological inhibition of ERK1/2 activation markedly attenuates the apela-induced inotropy. Analysis of samples from infarcted mouse hearts showed that expression of both apela and apelin receptor is induced in failing mouse hearts and correlate with left ventricular ejection fraction. Hence, we conclude that apela is present in the adult heart, is upregulated in post-infarction cardiac remodeling, and increases cardiac contractility in an ERK1/2-dependent manner.
This publication used ELABELA peptide (Cat#007-19), Apelin-13 (Cat#057-19) from Phoenix Pharmaceuticals.
Perjés Á, Kilpiö T, Ulvila J, et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res Cardiol. 2016;111(1):2.
Apelin and its G protein-coupled receptor (GPCR) have emerged as a key signalling pathway in the cardiovascular system. The peptide is a potent inotropic agent and vasodilator. Remarkably, a peptide, Elabela/Toddler, that has little sequence similarity to apelin, has been proposed as a second endogenous apelin receptor ligand and is encoded by a gene from a region of the genome previously classified as 'non-coding'. Apelin is downregulated in pulmonary arterial hypertension and heart failure. To replace the missing endogenous peptide, 'biased' apelin agonists have been designed that preferentially activate G protein pathways, resulting in reduced ?-arrestin recruitment and receptor internalisation, with the additional benefit of attenuating detrimental ?-arrestin signalling. Proof-of-concept studies support the clinical potential for apelin receptor biased agonists.
Yang P, Maguire JJ, Davenport AP. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci. 2015;36(9):560-7.
Apela (APJ early endogenous ligand, also known as elabela or toddler) is a recently discovered peptide hormone. Based on genetic studies in zebrafish, apela was found to be important for endoderm differentiation and heart development during embryogenesis. Although common phenotypes of apela and APJ null zebrafish during embryonic development suggested that apela interacts with the APJ receptor, kinetics of apela binding to APJ and intracellular signaling pathways for apela remain unknown. The role of apela in adults is also uncertain. Using a chimeric apela ligand, we showed direct binding of apela to APJ with high affinity (Kd=0.51nM) and the ability of apelin, the known peptide ligand for APJ, to compete for apela binding. Apela, similar to apelin, acts through the inhibitory G protein pathway by inhibiting forskolin-stimulated cAMP production and by inducing ERK1/2 phosphorylation. In adult rats, apela is expressed exclusively in the kidney, unlike the wide tissue distribution of apelin. In vivo studies demonstrated the ability of apela to regulate fluid homeostasis by increasing diuresis and water intake. Dose-response studies further indicated that apela induces 2- and 5-fold higher maximal responses than apelin ERK1/2 phosphorylation and diuresis/water intake, respectively. After designing an apela antagonist, we further demonstrated the role of endogenous ligand(s) in regulating APJ-mediated fluid homeostasis. Our results identified apela as a potent peptide hormone capable of regulating fluid homeostasis in adult kidney through coupling to the APJ-mediated Gi signaling pathway.
This publication used ELA products from Phoenix Pharmaceuticals.Deng C, Chen H, Yang N, Feng Y, Hsueh AJW. Apela Regulates Fluid Homeostasis by Binding to the APJ Receptor to Activate Gi Signaling. The Journal of Biological Chemistry. 2015;290(30):18261-18268. doi:10.1074/jbc.M115.648238.
A key step in the de novo formation of the embryonic vasculature is the migration of endothelial precursors, the angioblasts, to the position of the future vessels. To form the first axial vessels, angioblasts migrate towards the midline and coalesce underneath the notochord. Vascular endothelial growth factor (Vegf) has been proposed to serve as a chemoattractant for the angioblasts and to regulate this medial migration. Here we challenge this model and instead demonstrate that angioblasts rely on their intrinsic expression of Apelin receptors (Aplr, APJ) for their migration to the midline. We further show that during this angioblast migration Apelin receptor signaling is mainly triggered by the recently discovered ligand Elabela (Ela). As neither of the ligands Ela or Apelin (Apln) nor their receptors have previously been implicated in regulating angioblast migration, we hereby provide a novel mechanism for regulating vasculogenesis, with direct relevance to physiological and pathological angiogenesis.
Helker CS, Schuermann A, Pollmann C, et al. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. Elife. 2015;4
Over the past decade, high-throughput studies have identified many novel transcripts. While their existence is undisputed, their coding potential and functionality have remained controversial. Recent computational approaches guided by ribosome profiling have indicated that translation is far more pervasive than anticipated and takes place on many transcripts previously assumed to be non-coding. Some of these newly discovered translated transcripts encode short, functional proteins that had been missed in prior screens. Other transcripts are translated, but it might be the process of translation rather than the resulting peptides that serves a function. Here, we review annotation studies in zebrafish to discuss the challenges of placing RNAs onto the continuum that ranges from functional protein-encoding mRNAs to potentially non-functional peptide-producing RNAs to non-coding RNAs. As highlighted by the discovery of the novel signaling peptide Apela/ELABELA/Toddler, accurate annotations can give rise to exciting opportunities to identify the functions of previously uncharacterized transcripts.
Pauli A, Valen E, Schier AF. Identifying (non-)coding RNAs and small peptides: challenges and opportunities. Bioessays. 2015;37(1):103-12.
The identification of molecules controlling embryonic patterning and their functional analysis has revolutionized the fields of Developmental and Cell Biology. The use of new sequence information and modern bioinformatics tools has enriched the list of proteins that could potentially play a role in regulating cell behavior and function during early development. The recent application of efficient methods for gene knockout in zebrafish has accelerated the functional analysis of many proteins, some of which have been overlooked due to their small size. Two recent publications report on the identification of one such protein and its role in zebrafish embryogenesis. The protein, currently designated Apela, was shown to act as a secreted protein whose absence adversely affected various early developmental processes. Additional signaling proteins that have been identified in one of the studies are likely to open the way to unraveling hitherto unknown developmental pathways and have the potential to provide a more comprehensive understanding of known developmental processes.
Reichman-fried M, Raz E. Small proteins, big roles: the signaling protein Apela extends the complexity of developmental pathways in the early zebrafish embryo. Bioessays. 2014;36(8):741-5.
The secreted signals and signaling pathways that specify cell fate and pattern the body axes of early vertebrate embryos are generally well characterized, but the signals that trigger gastrulation, a major morphogenetic movement that internalizes the endoderm and mesoderm, remain largely uncharacterized. Pauli et al. identified Toddler (Tdl) as a secreted short peptide that is required for gastrulation in zebrafish embryos. The tdl transcript was present throughout the embryo during late blastula and early gastrula stages and was required only during these stages for proper gastrulation. Loss-of-function mutants had various endodermal and mesodermal defects, and the endodermal cells in these mutants migrated more slowly and shorter distances than endodermal cells in gastrulating wild-type embryos. Injection of 2 pg of tdl mRNA rescued the loss-of-function phenotype, but injection of 10 pg or more partially phenocopied loss-of-function mutations, indicating that the precise amount of Tdl activity was important for proper gastrulation. The Apelin receptor (APJ), a G protein–coupled receptor (GPCR) that is activated by the peptide Apelin, was present in mesendodermal cells, and its abundance peaked during gastrulation. Embryos lacking Apelin receptors phenocopied tdl mutants, and injection of Apelin mRNA, which is not normally present during gastrulation, rescued the tdl mutant phenotype. GPCRs are typically internalized upon activation, and expressing tdl in clones of cells in pregastrula embryos caused internalization of a fluorescently tagged version of the Apelin receptor in neighboring cells. This study identifies Tdl as an endogenous ligand for the Apelin receptor, activation of which is required for proper cell movements during zebrafish gastrulation. Human and mouse homologs of tdl were also identified, suggesting that this peptide may also function as a ligand for Apelin receptors in other species. Tdl (also called Elabela) was recently independently reported by Chng et al. to act as a ligand for the Apelin receptor during heart development in zebrafish.
Vanhook A. A secreted peptide promotes gastrulatin movements in Zebrafish. Sci Signal. 2014;EC46.
It has been assumed that most, if not all, signals regulating early development have been identified. Contrary to this expectation, we identified 28 candidate signaling proteins expressed during zebrafish embryogenesis, including Toddler, a short, conserved, and secreted peptide. Both absence and overproduction of Toddler reduce the movement of mesendodermal cells during zebrafish gastrulation. Local and ubiquitous production of Toddler promote cell movement, suggesting that Toddler is neither an attractant nor a repellent but acts globally as a motogen. Toddler drives internalization of G protein-coupled APJ/Apelin receptors, and activation of APJ/Apelin signaling rescues toddler mutants. These results indicate that Toddler is an activator of APJ/Apelin receptor signaling, promotes gastrulation movements, and might be the first in a series of uncharacterized developmental signals. Evidence indicates that ELA is expressed in human embryonic stem cells and adult human prostate and kidney. ELA may have cardioprotective and/or vasodilatory properties in humans and may play a role in cancer.
Pauli A, Norris ML, Valen E, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343(6172):1248636.

| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 007-32 | ELA (19-32) / prepro-ELA (41-54) (Rat) | 100 µg | $242 |
| 007-29 | ELA, N-terminal / prepro-ELA (23-39) (Human) | 100 µg | $230 |
| 007-21 | ELA, N-terminal / prepro-ELA (23-41) (Human) | 100 µg | $230 |
| B-007-27 | ELA-21 (Mouse) - Biotin Labeled | 20 µg | $444 |
| T-007-27 | ELA-21 (Mouse) - I-125 Labeled | 10 µCi | $1009 |
| RK-007-27 | ELA-21 (Mouse) - RIA Kit | 125 tubes | $926 |
| B-007-26 | ELA-21 (Rat) - Biotin Labeled | 20 µg | $444 |
| T-007-26 | ELA-21 (Rat) - I-125 Labeled | 10 µCi | $1009 |
| B-007-25 | ELA-32 (Human) - Biotin Labeled | 20 µg | $444 |
| B-G-007-19 | [pGlu1]-ELA-32 (Human) - Biotin Labeled Purified IgG | 100 µl | $635 |
Social Network Confirmation