
Abstract: Unlike severe acute respiratory syndrome(SARS) and Middle East respiratory syndrome MERS), a far greater proportion of world’s population has been afflicted by coronavirus disease 2019 (COVID-19). As fatalities still continue to soar, researchers scramble to re-purpose existing medications to tackle the disease. A growing number of pharmaceutical companies have embarked upon clinical trials on a litany of anti-viral drugs such as lopinavir/ritonavir (HIV-1 protease inhibitor), oseltamivir (influenza virus neuraminidase inhibitor), chloroquine phosphate (a quinoline compound with anti-malarial and anti-inflammatory properties), and remdesivir (a broad-spectrum inhibitor of viral RNA-dependent RNA polymerase), to mention only a few.
Memariani H, Memariani M. Therapeutic and prophylactic potential of anti-microbial peptides against coronaviruses. Ir J Med Sci. 2020;
Abstract: A safe, potent and broad-spectrum antiviral is urgently needed to combat emerging respiratory viruses. In light of the broad antiviral activity of β-defensins, we tested the antiviral activity of 11 peptides derived from mouse β-defensin-4 and found that a short peptide, P9, exhibited potent and broad-spectrum antiviral effects against multiple respiratory viruses in vitro and in vivo, including influenza A virus H1N1, H3N2, H5N1, H7N7, H7N9, SARS-CoV and MERS-CoV. The antiviral activity of P9 was attributed to its high-affinity binding to viral glycoproteins, as well as the abundance of basic amino acids in its composition. After binding viral particles through viral surface glycoproteins, P9 entered into cells together with the viruses via endocytosis and prevented endosomal acidification, which blocked membrane fusion and subsequent viral RNA release. This study has paved the avenue for developing new prophylactic and therapeutic agents with broad-spectrum antiviral activities.
Zhao H, Zhou J, Zhang K, et al. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci Rep. 2016;6:22008.
Objectives: To examine the effects and mechanisms of human neutrophil peptides in systemic infection and noninfectious inflammatory lung injury.Design: Prospective experimental study. Setting: University hospital-based research laboratory. Subjects: In vitro human cells and in vivo mouse models.Interventions: Wild-type (Friend virus B-type) and conditional leukocyte human neutrophil peptides transgenic mice were subjected to either sepsis induced by cecal ligation and puncture or acute lung injury by intratracheal instillation of hydrochloric acid followed by mechanical ventilation. Using human neutrophil peptides as bait, the basal cell adhesion molecule (CD239) and the purinergic P2Y purinoceptor 6 receptor were identified as the putative human neutrophil peptides receptor complex in human lung epithelial cells.Measurements and main results: In the cecal ligation and puncture sepsis model, Friend virus B-type mice exhibited higher systemic bacterial load, cytokine production, and lung injury than human neutrophil peptides transgenic mice. Conversely, an increased lung cytokine production was seen in Friend virus B-type mice, which was further enhanced in human neutrophil peptides transgenic mice in response to two-hit lung injury induced by hydrochloric acid and mechanical ventilation. The human neutrophil peptides-mediated inflammatory response was mediated through the basal cell adhesion molecule-P2Y purinoceptor 6 receptor signal pathway in human lung epithelial cells.Conclusions: Human neutrophil peptides are critical in host defense against infectious sepsis by their cationic antimicrobial properties but may exacerbate tissue injury when neutrophil-mediated inflammatory responses are excessive in noninfectious lung injury. Targeting the basal cell adhesion molecule/P2Y purinoceptor 6 signaling pathway may serve as a novel approach to attenuate the neutrophil-mediated inflammatory responses and injury while maintaining the antimicrobial function of human neutrophil peptides in critical illness.Wu J, Han B, Fanelli V, et al. Distinctive Roles and Mechanisms of Human Neutrophil Peptides in Experimental Sepsis and Acute Respiratory Distress Syndrome. Crit Care Med. 2018;46(9):e921-e927.
Abstract: Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is a highly pathogenic respiratory virus with mechanisms that may be driven by innate immune responses. Despite the effort of scientific studies related to this virus, Middle East Respiratory Syndrome (MERS) is still a public health concern. MERS-CoV infection has a high mortality rate, and to date, no therapeutic or vaccine has been discovered, that is effective in treating or preventing the disease. In this review, we summarize our understanding of the molecular and biological events of compounds acting as MERS-CoV inhibitors, the outcomes of existing therapeutic options and the various drugs undergoing clinical trials. Currently, several therapeutic options have been employed, such as convalescent plasma (CP), intravenous immunoglobulin (IVIG), monoclonal antibodies and repurposing of existing clinically approved drugs. However, these therapeutic options have drawbacks, thus the need for an alternative approach. The requirement for effective therapeutic treatment has brought the necessity for additional MERS treatments. We suggest that antimicrobial peptides (AMPs) may be used as alternative therapeutic agents against MERS-CoV infection. In addition, we propose the feasibility of developing effective agents by repurposing the existing and clinically approved anti-coronavirus and anti-viral peptide drugs.
Mustafa S, Balkhy H, Gabere MN. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): A review. J Infect Public Health. 2018;11(1):9-17.
Abstract: Human defensins are innate immune defense peptides with a remarkably broad repertoire of anti-pathogen activities. In addition to modulating immune response, inflammation, and angiogenesis, disintegrating bacterial membranes, and inactivating bacterial toxins, defensins are known to intercept various viruses at different stages of their life cycles, while remaining relatively benign towards human cells and proteins. Recently we have found that human defensins inactivate proteinaceous bacterial toxins by taking advantage of their low thermodynamic stability and acting as natural "anti-chaperones", i.e. destabilizing the native conformation of the toxins. In the present study we tested various proteins produced by several viruses (HIV-1, PFV, and TEV) and found them to be susceptible to destabilizing effects of human α-defensins HNP-1 and HD-5 and the synthetic θ-defensin RC-101, but not β-defensins hBD-1 and hBD-2 or structurally related plant-derived peptides. Defensin-induced unfolding promoted exposure of hydrophobic groups otherwise confined to the core of the viral proteins. This resulted in precipitation, an enhanced susceptibility to proteolytic cleavage, and a loss of viral protein activities. We propose, that defensins recognize and target a common and essential physico-chemical property shared by many bacterial toxins and viral proteins - the intrinsically low thermodynamic protein stability.
Kudryashova E, Koneru PC, Kvaratskhelia M, Strömstedt AA, Lu W, Kudryashov DS. Thermodynamic instability of viral proteins is a pathogen-associated molecular pattern targeted by human defensins. Sci Rep. 2016;6:32499.
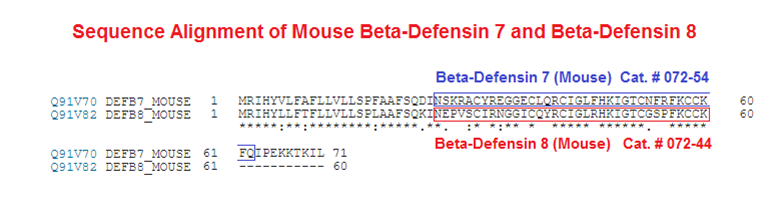
Abstract: Defensins are cationic and cysteine-rich peptides that play a crucial role in the host defense against microorganisms of many organisms by their capability to permeabilize bacterial membranes. The low sequence similarity among the members of the large mammalian beta-defensin family suggests that their antimicrobial activity is largely independent of their primary structure. To investigate to what extent these defensins share a similar fold, the structures of the two human beta-defensins, hBD-1 and hBD-2, as well as those of two novel murine defensins, termed mBD-7 and mBD-8, were determined by nuclear magnetic resonance spectroscopy. All four defensins investigated share a striking similarity on the level of secondary and tertiary structure including the lack of a distinct hydrophobic core, suggesting that the fold is mainly stabilized by the presence of three disulfide bonds. In addition to the overall shape of the molecules, the ratio of solvent-exposed polar and hydrophobic side chains is also very similar among the four defensins investigated. It is significant that beta-defensins do not exhibit a common pattern of charged and hydrophobic residues on the protein surface and that the beta-defensin-specific fold appears to accommodate a wide range of different amino acids at most sequence positions. In addition to the implications for the mode of biological defensin actions, these findings are of particular interest because beta-defensins have been suggested as lead compounds for the development of novel peptide antibiotics for the therapy of infectious diseases.
Bauer F, Schweimer K, Klüver E, et al. Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 2001;10(12):2470-9.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-072-37 | Defensin 2, beta (Human) - ELISA Kit | 96 wells | $570 |
| 072-54 | Defensin 7, Beta (Mouse) | 100 µg | $415 |
| 072-42 | Defensin-3, Beta (Human) | 100 µg | $415 |
| 072-41A | Defensin-4 , Beta (Human) | 100 µg | $474 |
| 072-41B | Defensin-4, Beta (Human) | 20 µg | $237 |
| 072-48 | Defensin 2, beta, synthetic (Human) | 100 µg | $306 |
| EK-072-38 | Defensin 3, beta (Human) - ELISA Kit | 96 wells | $570 |
| 072-53 | Defensin 1, beta, synthetic (Human) | 20 µg | $202 |
| H-072-53 | Defensin 1, beta, synthetic (Human) - Antibody | 100 µl | $571 |
| B-G-072-53 | Defensin 1, beta, synthetic (Human) - Biotin Labeled Purified IgG | 100 µl | $629 |
Social Network Confirmation