Although the accumulation of amyloidogenic proteins in neuroinflammatory conditions is generally considered pathologic, in a murine model of multiple sclerosis, amyloid-forming fibrils, comprised of hexapeptides, are anti-inflammatory. Whether these molecules modulate systemic inflammatory conditions remains unknown. We hypothesized that an amylin hexapeptide that forms fibrils can attenuate the systemic inflammatory response in a murine model of sepsis. To test this hypothesis, mice were pre-treated with either vehicle or amylin hexapeptide (20 μg) at 12 hours and 6 hours prior to intraperitoneal (i.p.) lipopolysaccharide (LPS, 20 mg/kg) administration. Illness severity and survival were monitored every 6 hours for 3 days. Levels of pro- (IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-10) cytokines were measured via ELISA at 1, 3, 6, 12, and 24 hours after LPS (i.p.). As a metric of lung injury, pulmonary artery endothelial cell (PAEC) barrier function was tested 24 hours after LPS administration by comparing lung wet-to-dry ratios, Evan's blue dye (EBD) extravasation, lung histology and caspase-3 activity. Compared to controls, pretreatment with amylin hexapeptide significantly reduced mortality (p<0.05 at 72 h), illness severity (p<0.05), and pro-inflammatory cytokine levels, while IL-10 levels were elevated (p<0.05). Amylin pretreatment attenuated LPS-induced lung injury, as demonstrated by decreased lung water and caspase-3 activity (p<0.05, versus PBS). Hence, in a murine model of systemic inflammation, pretreatment with amylin hexapeptide reduced mortality, disease severity, and preserved lung barrier function. Amylin hexapeptide may represent a novel therapeutic tool to mitigate sepsis severity and lung injury.
Mahapatra S, Ying L, Ho PP et al., PLoS One. 2018 Jul 10;13(7):e0199206. doi: 10.1371/journal.pone.0199206. eCollection 2018.
The islet amyloid polypeptide (IAPP) or amylin maintains a key role in metabolism. This 37-residues-peptide could form pancreatic amyloids, which are a characteristic feature of diabetes mellitus type 2. However, some species do not form amyloid fibril structures. By employing a biomimetic approach, we generated an extensive panel of optimized sequences of IAPP, which could drastically reduce aggregation propensity. A structural and cellular characterization analysis was performed on the C-terminal domain with the highest aggregation propensity. This allowed the observation of an aggregative phenomenon dependent of the lipid environment. Evaluation of the new F23R variant demonstrated inhibition of β-sheet structure and, therefore, amyloid formation on the native C-terminal, phenomenon that was associated with functional optimization in calcium and cholesterol management coupled with the optimization of insulin secretion by beta cells. When F23R variant was evaluated in microglia cells, a model of amyloidosis, cytotoxic conditions were not registered. In addition, it was found that C-terminal sequences of IAPP could modulate cholesterol metabolism in hepatocytes through regulation of SREBP-2, apoA-1, ABCA1, and LDLR, mechanism that may represent a new function of IAPP on the metabolism of cholesterol, increasing the LDL endocytosis in hepatocytes. Optimized sequences with only one residue modification in the C-terminal core aggregation could diminish β-sheet formation and represent a novel strategy adaptable to other pharmacological targets. Our data suggest a new IAPP function associated with rearrangements on metabolism of cholesterol in hepatocytes.
Pulido-capiz A, Díaz-molina R, Martínez-navarro I, et al. Modulation of Amyloidogenesis Controlled by the C-Terminal Domain of Islet Amyloid Polypeptide Shows New Functions on Hepatocyte Cholesterol Metabolism. Front Endocrinol (Lausanne). 2018;9:331.
The abnormal fibrillogenesis of amyloid peptides, such as amyloid fibril and senior amyloid plaques, is associated with the pathogenesis of many amyloid diseases. Hence, modulation of amyloid assemblies is related to the possible pathogenesis of some diseases. Some two-dimensional nanomaterials, that is, graphene oxide, tungsten disulfide, exhibit strong modulation effects on the amyloid fibrillogenesis. Herein, the modulation effect of molybdenum disulfide on two amyloid peptide assemblies based on the label-free techniques is presented, including quartz crystal microbalance (QCM), AFM, and CD spectroscopy. MoS2 presents different modulating effects on the assembly of amyloid-β peptide (33-42) [Aβ (33-42)] and amylin (20-29) (Phoenix, Cat. #017-09), mainly owing to the distinct affinity between amyloid peptides and MoS2. This is to our knowledge the first report of MoS2 as a modulator for amyloid aggregation. It enriches the variety of 2D nanomodulators of amyloidfibrillogenesis and explains the mechanism for the self-assembly of amyloid peptides, and expands the applications of MoS2 in biology.
This publication used a peptide from Phoenix Pharmaceuticals for assembly dynamics study of hlAPP.
Wang J, Liu L, Ge D, et al. Differential Modulating Effect of MoS on Amyloid Peptide Assemblies. Chemistry. 2018;24(14):3397-3402.
The misfolding and subsequent assembly of proteins and peptides into insoluble amyloid structures play important roles in the development of numerous diseases. The dynamics of self-assembly and the morphology of the resulting aggregates critically depend on various environmental factors and especially on the presence of interfaces. Here, we show in detail how the presence of surfaces with different physicochemical properties influences the assembly dynamics and especially the aggregate morphology of hIAPP(20-29), an amyloidogenic fragment of the peptide hormone human islet amyloid polypeptide (hIAPP), which is involved in the development of type 2 diabetes. Time-lapse atomic force microscopy is employed to study the assembly dynamics of hIAPP(20-29) (Phoenix, Cat. #017-09) and the morphology of the resulting aggregates in bulk solution as well as at hydrophilic and hydrophobic model surfaces. We find that the presence of hydrophilic mica surfaces promotes fibrillation when compared with the assembly in bulk solution and results in a more pronounced polymorphism. Three fibrillar species are found to coexist on the mica surface, that is, straight, coiled, and ribbon-like fibrils, whereas only the straight and coiled fibrils are observed in bulk solution after comparable incubation times. In addition, the straight and coiled fibrils assembled at the mica surface have significantly different dimensions compared with those assembled in bulk solution. The three fibrillar species found on the mica surface most likely form independently by lateral association of arbitrary numbers of protofibrils with about 2 nm height. On hydrophobic hydrocarbon surfaces, fibrillation is retarded but not completely suppressed, in contrast to previous observations for full-length hIAPP(1-37). Our results show that peptide-surface interactions may induce diverse, peptide-specific alterations of amyloid assembly dynamics and fibrillar polymorphism. They may therefore contribute to a deeper understanding of the molecular processes that govern amyloid aggregation at different surfaces.
This publication used a peptide from Phoenix Pharmaceuticals for assembly dynamics study of hlAPP.
Hajiraissi R, Giner I, Grundmeier G, Keller A. Self-Assembly, Dynamics, and Polymorphism of hIAPP(20-29) Aggregates at Solid-Liquid Interfaces. Langmuir. 2017;33(1):372-381.
Amyloid fibrils composed of peptides as short as six amino acids are therapeutic in experimental autoimmune encephalomyelitis (EAE), reducing paralysis and inflammation, while inducing several pathways of immune suppression. Intraperitoneal injection of fibrils selectively activates B-1a lymphocytes and two populations of resident macrophages (MΦs), increasing IL-10 production, and triggering their exodus from the peritoneum. The importance of IL-10-producing B-1a cells in this effective therapy was established in loss-of-function experiments where neither B-cell-deficient (μMT) nor IL10(-/-) mice with EAE responded to the fibrils. In gain-of-function experiments, B-1a cells, adoptively transferred to μMT mice with EAE, restored their therapeutic efficacy when Amylin 28-33 was administered. Stimulation of adoptively transferred bioluminescent MΦs and B-1a cells by amyloid fibrils resulted in rapid (within 60 min of injection) trafficking of both cell types to draining lymph nodes. Analysis of gene expression indicated that the fibrils activated the CD40/B-cell receptor pathway in B-1a cells and induced a set of immune-suppressive cell-surface proteins, including BTLA, IRF4, and Siglec G. Collectively, these data indicate that the fibrils activate B-1a cells and F4/80(+) MΦs, resulting in their migration to the lymph nodes, where IL-10 and cell-surface receptors associated with immune-suppression limit antigen presentation and T-cell activation. These mechanisms culminate in reduction of paralytic signs of EAE.
Kurnellas MP, Ghosn EE, Schartner JM, et al. Proc Natl Acad Sci USA. 2015;112(49):15016-23.
In cats, the incidence of obesity and diabetes is increasing, and little is known about specific aspects of the endocrine control of food intake in this species. Recent data suggest that ghrelin has an important role in the control of insulin secretion and vice versa, but this role has never been demonstrated in cats. Here we aimed to improve our understanding about the relationship between insulin, amylin and ghrelin secretion in responseto a nutrient load in overweight cats. After a 16 h fast, weekly, six overweight male cats underwent randomly one of the four testing sessions: saline, glucose, arginine and TAG. All solutions were isoenergetic and isovolumic, and were injected intravenously as a bolus. Glucose, insulin, acylated ghrelin (AG), amylin and prolactin were assayed in plasma before and 10, 20, 40, 60, 80 and 100 min after the nutrient load. A linear mixed-effects model was used to assess the effect of bolus and time on the parameters. A parenteral bolus of glucose or arginine increased insulin and ghrelin concentrations in cats. Except for with the TAG bolus, no suppression of ghrelin was observed. The absence of AG suppression after the intravenous load of arginine and glucose may suggest: (1) that some nutrients do not promote satiation in overweight cats; or that (2) AG may be involved in non-homeostatic consumption mechanisms. However, the role of ghrelin in food reward remains to be assessed in cats.
This publication used an Amylin RIA kit (#RK-017-03) from Phoenix Pharmaceuticals.
Martin LJ, Lutz TA, Daumas C, et al. Acute hormonal response to glucose, lipids and arginine infusion in overweight cats. J Nutr Sci. 2014;3:e8.
Diet-induced obesity is a lifestyle-associated medical condition that increases the risk of developing cardiovascular disease, type 2 diabetes and certain types of cancer. Here we report the design of a closed-loop genetic circuit that constantly monitors blood fatty acid levels in the setting of diet-associated hyperlipidemia and coordinates reversible and adjustable expression of the clinically licensed appetite-suppressing peptide hormone pramlintide. Grafting of the peroxisome proliferator-activated receptor-? onto the phloretin-responsive repressor TtgR produces a synthetic intracellular lipid-sensing receptor (LSR) that reversibly induces chimeric TtgR-specific promoters in a fatty acid-adjustable manner. Mice with diet-induced obesity in which microencapsulated cells engineered for LSR-driven expression of pramlintide are implanted show significant reduction in food consumption, blood lipid levels and body weight when put on a high-fat diet. Therapeutic designer circuits that monitor levels of pathologic metabolites and link these with the tailored expression of protein pharmaceuticals may provide new opportunities for the treatment of metabolic disorders.
This publication used an Amylin EIA kit (#EK-017-11) from Phoenix Pharmaceuticals.
Rössger K, Charpin-el-hamri G, Fussenegger M. A closed-loop synthetic gene circuit for the treatment of diet-induced obesity in mice. Nat Commun. 2013;4:2825.
CONTEXT: Anorexia nervosa, characterized by extreme low body weight due to reduced nutrient intake, is associated with severe bone loss. Peptide hormones, including amylin, GIP, and GLP2, are released immediately after nutrient intake and may be involved in the regulation of boneturnover.
OBJECTIVE: To investigate fasting levels of amylin, GIP, and GLP2 and their relationships with bone mineral density (BMD) in women with anorexia nervosa compared to healthy controls.
DESIGN: Cross-sectional.
SETTING: Clinical Research Center.
STUDY PARTICIPANTS: 15 women with anorexia nervosa and 16 healthy controls.
INTERVENTION: None.
MAIN OUTCOME MEASURES: Fasting serum amylin, GIP, and GLP2, and BMD.
RESULTS: Women with anorexia nervosa had significantly lower fasting serum amylin and GIP levels than healthy controls. Fasting serum GLP2 levels were not significantly different between groups. Fasting amylin levels were positively associated with BMD and Z-score at the PA spine, total hip, and femoral neck. Fasting amylin levels were also positively associated with weight and percent fat; after controlling for these variables, amylin was still a significant predictor of BMD and Z-score at the femoral neck and of Z-score at the total hip. In the anorexia nervosa group, there was a trend toward an inverse association between amylin and C-terminal telopeptide (CTX) levels (R=-0.47, p=0.08). GIP and GLP2 levels did not predict BMD at any site.
CONCLUSION: Decreased secretion of amylin may be a mechanism through which reduced nutrient intake adversely affects BMD in anorexia nervosa.
This publication used an Amylin EIA kit (#EK-017-11) from Phoenix Pharmaceuticals.
Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone. 2010;46(3):796-800.
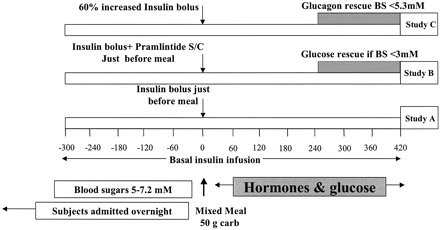
Postprandial hyperglycemia and preprandial hypoglycemia contribute to poor glycemic control in type 1 diabetes. We hypothesized that postprandial glycemic excursions could be normalized in type 1 diabetes by suppressing glucagon with pramlintide acetate in the immediate postprandial period and supplementing glucagon in the late postprandial period. A total of 11 control subjects were compared with 8 type 1 diabetic subjects on insulin pump therapy, using the usual insulin bolus-to-carbohydrate ratio during a standard liquid meal. Type 1 diabetic subjects were then randomized to two open-labeled studies. On one occasion, type 1 diabetic subjects received a 60% increase in the insulin bolus-to-carbohydrate ratio with minidose glucagon rescue injections, and on the other occasion type 1 diabetic subjects received 30-45 microg pramlintide with their usual insulin bolus-to-carbohydrate ratio. Glucose, glucagon, amylin (pramlintide), and insulin concentrations were measured for 420 min. The plasma glucose area under the curve (AUC) for 0-420 min was lower in control versus type 1 diabetic subjects (316 +/- 5 vs. 929 +/- 18 mg x h(-1) x dl(-1), P < 0.0001). Pramlintide, but not an increase in insulin, reduced immediate postprandial hyperglycemia (AUC(0-180 min) 470 +/- 43 vs. 434 +/- 48 mg x h(-1) x dl(-1), P < 0.01). Pramlintide administration suppressed glucagon (P < 0.02), and glucagon injections prevented late hypoglycemia with increased insulin. In summary, in type 1 diabetes, glucagon modulation with pramlintide as an adjunct to insulin therapy may prove beneficial in controlling postmeal glycemic swings.
Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes. 2005;54(4):1100-7.
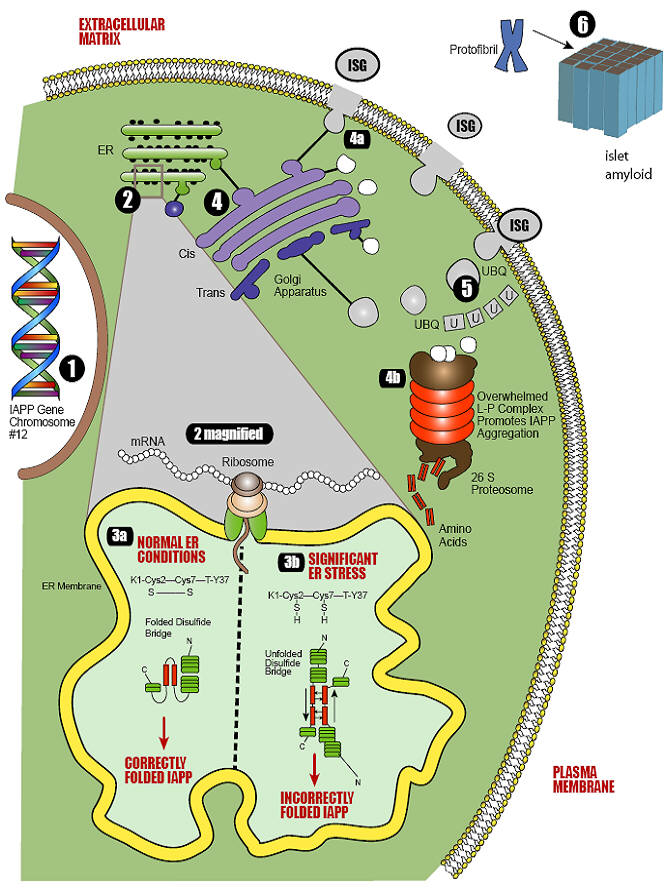
Conformational diseases are conditions that arise from the dysfunctional aggregation of proteins in non-native conformations. Type 2 diabetes mellitus can be defined as a conformational disease because a constituent beta cell protein, islet amyloid polypeptide, undergoes a change in tertiary structure followed by self-association and tissue deposition. Type 2 diabetes mellitus is associated with multiple metabolic derangements that result in the excessive production of reactive oxygen species and oxidative stress. These reactive oxygen species set in motion a host of redox reactions which can result in unstable nitrogen and thiol species that contribute to additional redox stress. The ability of a cell to deal with reactive oxygen species and oxidative stress requires functional chaperones, antioxidant production, protein degradation and a cascade of intracellular events collectively known as the unfolded protein response. It is known that beta cells are particularly susceptible to perturbations in this quality control system and that reactive oxygen species play an important role in the development and/or progression of diabetes mellitus. Oxidative stress and increased insulin production contribute to endoplasmic reticulum stress, protein misfolding, and induction of the unfolded protein response. As the cell's quality control system becomes overwhelmed, conformational changes occur to islet amyloid polypeptide intermediates, generating stable oligomers with an anti-parallel crossed beta-pleated sheet structure that eventually accumulate as space-occupying lesions within the islets. By approaching type 2 diabetes mellitus as a conformational disease in which there is a structural transition from physiological protein to pathological protein, it is possible that the relentless nature of disease progression can be understood in relation to other conformational diseases.
Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6(4):287-302
BACKGROUND: Amylin is a novel 37 amino acid peptide hormone that is co-secreted with insulin from the pancreas in response to food intake. As a potent inhibitor of gastric emptying it plays an important role in the control of carbohydrate absorption. Feed intolerance is common in infants of diabetic mothers (IDM).
AIMS: To establish a normal range of amylin levels in healthy neonates, and to determine whether serum amylin levels are raised in IDM.
METHODS: A serial sample of 221 infants > or =28 weeks gestation was enrolled prior to delivery over a 12 month period. Blood samples collected immediately after birth (umbilical cord), and at the routine Guthrie test were analysed for amylin and insulin levels.
RESULTS: Amylin levels in umbilical cord (n = 181) and Guthrie samples (n = 33) of healthy infants were 5.7 (3.0-9.1) and 6.9 (2.9-9.0) pmol/l respectively. IDM had significantly raised amylin levels in both cord (n = 31; 32.7 pmol/l, 25.9-48.1) and Guthrie samples (n = 8; 18.1 pmol/l, 15.3-23.6). Amylin correlated positively with insulin (n = 42; r = 0.67; 95% CI 0.4 to 0.81), birth weight (r = 0.22; 95% CI 0.08 to 0.36), and gestation (r = 0.18; 95% CI 0.03 to 0.32). Umbilical cord venous amylin levels showed agreement with arterial cord amylin levels (n = 34, mean bias -0.2, 95% CI 3.1 to -3.6).
CONCLUSIONS: Amylin levels are significantly increased in the umbilical cord and Guthrie blood samples in IDM.
Kairamkonda V, Deorukhkar A, Coombs R, Fraser R, Mayer T. Amylin peptide levels are raised in infants of diabetic mothers. Arch Dis Child. 2005;90(12):1279-82.
The causes of cerebral accumulation of amyloid ß-protein (Aß) in most cases of Alzheimer’s disease (AD) remain unknown. We recently found that homozygous removal of the insulin-degrading enzyme (IDE) gene in mice results in an early and marked elevation of cerebral Aß. Both genetic linkage and allelic association in the IDE region of chromosome 10 have been reported in families with late-onset AD. For IDE to remain a valid candidate gene for late-onset AD on functional grounds, it must be shown that partial loss of function of IDE can still alter Aß degradation, but without causing early, severe elevation of brain Aß. Here, we show that naturally occurring IDE missense mutations in a well-characterized rat model of type 2 diabetes mellitus (DM2) result in decreased catalytic efficiency and a significant 15 to 30% deficit in the degradation of both insulin and Aß. Endogenously secreted Aß40 and Aß42 are significantly elevated in primary neuronal cultures from animals with the IDE mutations, but there is no increase in steady-state levels of rodent Aß in the brain up to age 14 months. We conclude that naturally occurring, partial loss-of-function mutations in IDE sufficient to cause DM2 also impair neuronal regulation of Aß levels, but the brain can apparently compensate for the partial deficit during the life span of the rat. Our findings have relevance for the emerging genetic evidence suggesting that IDE may be a late-onset AD-risk gene, and for the epidemiological relationships among hyperinsulinemia, DM2, and AD.
Farris W, Mansourian S, Leissring MA, et al. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol. 2004;164(4):1425-34.
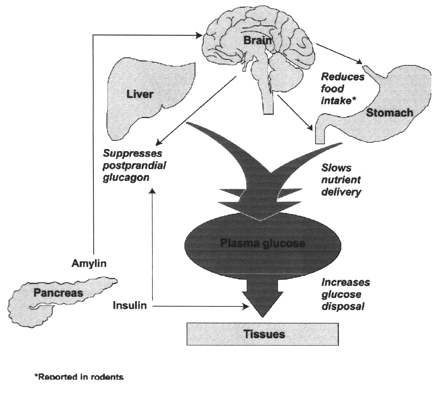
Amylin is a peptide hormone that is cosecreted with insulin from the pancreatic beta-cell and is thus deficient in diabetic people. It inhibits glucagon secretion, delays gastric emptying, and acts as a satiety agent. Amylin replacement could therefore possibly improve glycemic control in some people with diabetes. However, human amylin exhibits physicochemical properties predisposing the peptide hormone to aggregate and form amyloid fibers, which may play a part in beta-cell destruction in type 2 diabetes. This obviously makes it unsuitable for pharmacological use. A stable analog, pramlintide, which has actions and pharmacokinetic and pharmacodynamic properties similar to the native peptide, has been developed. The efficacy and safety of pramlintide administration has been tested in a vast number of clinical trials. Approximately 5,000 insulin-treated patients have received pramlintide and approximately 250 for > or =2 years. The aims of this review are to 1) briefly describe actions of amylin as demonstrated in animal and human models and 2) primarily review results from clinical trials with the amylin analog pramlintide.
Schmitz O, Brock B, Rungby J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes. 2004;53 Suppl 3:S233-8.
Islet amyloid deposition is a pathogenic feature of type 2 diabetes, and these deposits contain the unique amyloidogenic peptide islet amyloid polypeptide. Autopsy studies in humans have demonstrated that islet amyloid is associated with loss of beta-cell mass, but a direct role for amyloid in the pathogenesis of type 2 diabetes cannot be inferred from such studies. Animal studies in both spontaneous and transgenic models of islet amyloid formation have shown that amyloid forms in islets before fasting hyperglycemia and therefore does not arise merely as a result of the diabetic state. Furthermore, the extent of amyloid deposition is associated with both loss of beta-cell mass and impairment in insulin secretion and glucose metabolism, suggesting a causative role for islet amyloid in the islet lesion of type 2 diabetes. These animal studies have also shown that beta-cell dysfunction seems to be an important prerequisite for islet amyloid formation, with increased secretory demand from obesity and/or insulin resistance acting to further increase islet amyloid deposition. Recent in vitro studies suggest that the cytotoxic species responsible for islet amyloid-induced beta-cell death are formed during the very early stages of islet amyloid formation, when islet amyloid polypeptide aggregation commences. Interventions to prevent islet amyloid formation are emerging, with peptide and small molecule inhibitors being developed. These agents could thus lead to a preservation of beta-cell mass and amelioration of the islet lesion in type 2 diabetes.
Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629-43.
Many insulin-treated diabetic patients still fail to achieve optimal glycemic control and continue to experience problems with hypoglycemia, weight gain, and postprandial hyperglycemia. Adjunctive therapy with pramlintide, a synthetic analog of the human amylin hormone, facilitates a significant improvement of postprandial and overall glycemic control in patients with either type 1 or type 2 diabetes without an increased risk of hypoglycemia or weight gain.
Buse et al. Clinical Diabetes 20:137-144, 2002
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-017-11 | Amylin / IAPP (Rat, Mouse) - EIA Kit | 96 wells | $570 |
| 017-03 | Amylin / IAPP (Human) | 100 µg | $133 |
| EK-017-03 | Amylin / IAPP (Human) - EIA Kit | 96 wells | $570 |
| 017-11 | Amylin / IAPP (Rat, Mouse) | 500 µg | $336 |
| FEK-017-06 | Amylin / IAPP (1-13) (Human) - Fluorescent EIA Kit | 96 wells | $624 |
| 017-06 | Amylin / IAPP (1-13) (Human) | 200 µg | $102 |
| 017-07 | [Tyr0]-Amylin / IAPP (1-13) (Human) | 100 µg | $336 |
| H-017-06 | Amylin / IAPP (1-13) (Human) - Antibody | 100 µl | $279 |
| EK-017-06 | Amylin / IAPP (1-13) (Human) - EIA Kit | 96 wells | $570 |
| T-017-07 | [Tyr0]-Amylin / IAPP (1-13) (Human) - I-125 Labeled | 10 µCi | $1082 |
Social Network Confirmation