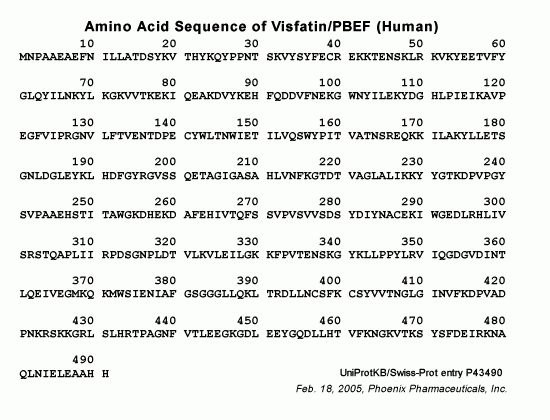
A New Natural Insulin-mimetic Adipokine
Abstract
Are all fats created equal?
The interplay of factors that impair insulin signaling to cause type 2 diabetes is unclear, but one idea is that visceral fat might produce them. Visfatin, a protein previously known for its effect on immune cells, may be one of these factors. The last decade has revolutionized our view of adipose tissue, transforming it from an inert storage depot into an important dynamic endocrine organ, secreting a number of adipokines—hormones produced by adipose tissue that regulate metabolism and energy homeostasis. More recently, new research has challenged the idea that adipose deposits represent a single aggregate functional entity.
Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance.
Visfatin, a new adipokine, facilitates adipogenesis and has insulin-mimetic properties. We aimed to investigate the plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus (T2DM) and impaired glucose tolerance (IGT), who had no obesity or hypertension. Twenty-two patients with T2DM, 18 subjects with IGT and 40 healthy controls were enrolled. Visfatin levels were measured along with the BMI, blood pressure, lipids, glucose, insulin, adiponectin and hsCRP levels, and HOMA-IR indexes. Age, sex and BMI were similar in all groups. Visfatin levels were higher in the diabetic group than the controls (p=0.01). There was no significant difference in the visfatin levels between the T2DM and IGT groups as well as IGT group and healthy controls. Plasma visfatin concentrations did not differ between men and women. Visfatin levels did not correlate with BMI, blood pressure, plasma adiponectin, insulin, hsCRP, glucose and lipid levels or HOMA-IR indexes in the three groups. These results indicate that hyperglycemia causes an increase in plasma visfatin levels and, as in people with T2DM but not with IGT, this increase gets more prominent as the glucose intolerance worsens.
Dogru et al. Diabetes Res Clin Pract. 2007 Apr;76(1):24-9.
Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans.
CONTEXT AND OBJECTIVE: Visfatin is a peptide suggested to play a role in glucose homeostasis. In the present study, we investigated the role of genetic variation in the visfatin gene in the pathophysiology of obesity/type 2 diabetes mellitus (T2DM). DESIGN: The visfatin gene (PBEF1) was sequenced in DNA samples from 24 nonrelated Caucasian subjects. Identified genetic variants were used for association analyses of T2DM in a case-control study (503 diabetic subjects and 476 healthy controls) and T2DM-related traits in 626 nondiabetic subjects. The effect of genetic variation in the visfatin gene on its mRNA expression in a subgroup of 157 nondiabetic subjects with measurements of visfatin mRNA expression in visceral and sc fat depots was also analyzed.
RESULTS: Seven single-nucleotide polymorphisms (SNPs) and one insertion/deletion were identified. Three SNPs (rs9770242, -948G–>T, rs4730153) that were representatives of their linkage disequilibrium groups were genotyped in Caucasians from Germany with a wide range of body fat distribution and insulin sensitivity for association analyses. No association of T2DM with any of the genotyped SNPs or their haplotypes was found. However, the ratio of visceral/sc visfatin mRNA expression was associated with all three genetic polymorphisms (P < 0.05). Moreover, the -948G–>T variant was associated with 2-h plasma glucose and fasting insulin concentrations (P < 0.05) in nondiabetic subjects.
CONCLUSIONS: In conclusion, our data suggest that genetic variation in the visfatin gene may have a minor effect on visceral and sc visfatin mRNA expression profiles but does not play a major role in the development of obesity or T2DM.
Böttcher Y, Teupser D, Enigk B, et al. Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans. J Clin Endocrinol Metab. 2006;91(7):2725-31.
Visfatin is an adipokine, but it is not regulated by thiazolidinediones.
CONTEXT: Visfatin was recently reported to be expressed in human adipose tissue and to exert insulin-mimicking effects. OBJECTIVE: The objective of this study was to examine whether visfatin is a true adipokine and is expressed in isolated fat cells. We also examined whether visfatin is regulated by thiazolidinediones and, thus, can contribute to the ability of these agents to improve insulin sensitivity.
DESIGN: This was an open-labeled drug therapy trial.
SETTING: This study was performed at a university hospital.
PATIENTS: Seven newly diagnosed and previously untreated type 2 diabetic patients and six healthy individuals with reduced insulin sensitivity participated in the study. INTERVENTION: Pioglitazone therapy (30-45 mg/d) was given for 3-4 wk.
MAIN OUTCOME MEASURES: Serum and adipose tissue mRNA levels of visfatin and adiponectin were the main outcome measures.
RESULTS: Visfatin mRNA is expressed in both adipose tissue and isolated adipocytes. Treatment with thiazolidinediones for 3-4 wk did not alter the gene expression or circulating levels of visfatin in either nondiabetic or the diabetic individuals, whereas adiponectin increased significantly.
CONCLUSION: The present study shows that visfatin is a true adipokine, but it is not regulated by TZD and, thus, is unlikely to contribute to the insulin-sensitizing actions of these drugs.
Hammarstedt A, Pihlajamäki J, Rotter sopasakis V, et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91(3):1181-4
Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus.
Visfatin (also known as pre-B cell colony-enhancing factor or PBEF) is a cytokine that is highly expressed in visceral fat and whose blood levels correlate with obesity. Originally isolated as a secreted factor that promotes the growth of B cell precursors and recently found to act as an insulin analog on the insulin receptor, its pathophysiological role in humans remains largely unknown.
OBJECTIVES: In this study we investigated whether plasma visfatin level is altered in patients with type 2 diabetes mellitus (T2DM).
DESIGN AND PATIENTS: Plasma visfatin as well as adiponectin and resistin concentrations were measured through ELISA in type 2 diabetic and nondiabetic subjects.
RESULTS: A total of 61 patients with T2DM and 59 sex- and age-matched nondiabetic subjects were studied. Plasma visfatin was found to be elevated in patients with T2DM (31.9 +/- 31.7 vs. 15.8 +/- 16.7 ng/ml, P = 0.002). In contrast, adiponectin was decreased (4.3 +/- 2.5 vs. 30.8 +/- 10.3 microg/ml, P < 0.001), whereas plasma resistin level did not differ between the groups. Increasing concentrations of visfatin were independently and significantly associated with T2DM. Multiple logistic regression analysis revealed visfatin as an independent association factor for T2DM, even after full adjustment of known biomarkers. The association between adiponectin and T2DM was no longer significant after adjustments for body mass index or waist to hip ratio. In a multiple linear regression analysis, only waist to hip ratio was independently associated with plasma visfatin level.
CONCLUSION: Our results indicate that visfatin may play a role in the pathogenesis of T2DM.
Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(1):295-9
Increased visfatin concentrations in women with gestational diabetes mellitus.
The recently discovered adipocytokine visfatin has insulin-like properties. It lowers blood glucose and improves insulin sensitivity; however, clinical data on visfatin are limited. To evaluate the role of visfatin in GDM (gestational diabetes mellitus), we determined visfatin levels in women with GDM and in healthy pregnant controls. Furthermore, visfatin concentrations were investigated longitudinally during pregnancy and after delivery in a subgroup of women with GDM. Blood for measurement of visfatin and metabolic parameters was obtained from 64 women with GDM [median week of gestation, 34 (interquartile range, 27-36) weeks] and 30 healthy pregnant controls [median week of gestation, 34 (interquartile range, 28-36) weeks]. In a subgroup of 24 women with GDM, visfatin, leptin and metabolic parameters were investigated twice during pregnancy (28-30 and 38-40 weeks of gestation) and 2 weeks after delivery. In the cross-sectional analysis, median visfatin levels were significantly elevated in women with GDM [64.0 (interquartile range, 50.9-74.8) ng/ml] compared with controls [46.0 (interquartile range, 36.9-54.6) ng/ml; P<0.0001]. In women with GDM, visfatin correlated with week of gestation at the time of blood draw (R=0.35, P=0.005). No association with fasting glucose, insulin, homoeostasis model assessment-insulin resistance or body mass index was observed. According to the longitudinal analysis, visfatin increased during pregnancy (P=0.002) and rose further after delivery (P=0.014), whereas leptin and insulin levels decreased after parturition (both P<0.001). In conclusion, visfatin is elevated in women with GDM and increases during the course of pregnancy as well as after delivery. Furthermore, visfatin shows no association with insulin and leptin in women with GDM.
Krzyzanowska K, Krugluger W, Mittermayer F, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci. 2006;110(5):605-9.
Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding.
CONTEXT: The insulin-mimetic adipocytokine visfatin has been linked to obesity. The influence of weight loss on plasma visfatin concentrations in obese subjects is unknown yet.
OBJECTIVES: In this study we investigated whether plasma visfatin concentrations are altered by weight loss in patients with obesity.
DESIGN AND PATIENTS: In a prospective study, fasting plasma visfatin, leptin, and adiponectin concentrations were measured before and 6 months after gastric banding in 31 morbidly obese patients aged 40 +/- 11 yr with a body mass index (BMI) of 46 +/- 5 kg/m(2). Fourteen healthy subjects aged 29 +/- 5 yr with a BMI less than 25 kg/m(2) served as controls.
RESULTS: Visfatin plasma concentrations were markedly elevated in obese subjects (0.037 +/- 0.008 microg/ml), compared with controls (0.001 +/- 0.000 microg/ml, P < 0.001). Gastric banding reduced BMI to 40 +/- 5 kg/m(2), visfatin to 19.2 +/- 10.9 ng/ml, and leptin from 39.0 +/- 12.4 to 29.7 +/- 10.0 ng/ml and increased adiponectin from 0.015 +/- 0.007 to 0.017 +/- 0.007 microg/ml (all P < 0.05) after 6 months. Insulin sensitivity as estimated by the homeostasis model assessment insulin resistance index was unchanged from 5.8 +/- 3.1 to 4.6 +/- 1.9 (P = 0.13), but individual changes of insulin resistance and visfatin were significantly associated (P < 0.05, r = -0.43). CONCLUSIONS: Elevated plasma visfatin concentrations in morbidly obese subjects are reduced after weight loss. This may be related to changes in insulin resistance over time.
Krzyzanowska K, Krugluger W, Mittermayer F, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci. 2006;110(5):605-9
Plasma visfatin concentrations and fat depot-specific mRNA expression in humans.
Visceral and subcutaneous adipose tissue display important metabolic differences that underlie the association of visceral obesity with obesity-related cardiovascular and metabolic alterations. Recently, visfatin was identified as an adipokine, which is predominantly secreted from visceral adipose tissue both in humans and mice. In this study, we examined whether visfatin plasma concentrations (using enzyme immunosorbent assay) and mRNA expression (using RT-PCR) in visceral and subcutaneous fat correlates with anthropometric and metabolic parameters in 189 subjects with a wide range of obesity, body fat distribution, insulin sensitivity, and glucose tolerance. Visfatin plasma concentration correlates positively with the visceral visfatin mRNA expression (r(2) = 0.17, P < 0.0001), BMI (r(2) = 0.062, P = 0.004), percent body fat (r(2) = 0.048, P = 0.01), and negatively with subcutaneous visfatin mRNA expression (r(2) = 0.18, P < 0.0001). However, in a subgroup of 73 individuals, in which visceral fat mass was calculated from computed tomography scans, there was no correlation between plasma visfatin concentrations and visceral fat mass. We found no significant correlation between visfatin plasma concentrations and parameters of insulin sensitivity, including fasting insulin, fasting plasma glucose concentrations, and the glucose infusion rate during the steady state of an euglycemic-hyperinsulinemic clamp independent of percent body fat. Visfatin gene expression was not different between visceral and subcutaneous adipose tissue in the entire study group nor in selected subgroups. We found a significant correlation between visceral visfatin gene expression and BMI (r(2) = 0.06, P = 0.001) and percent body fat (measured using dual-energy X-ray absorptiometry) (r(2) = 0.044, P = 0.004), whereas no significant association between BMI or percent body fat and subcutaneous visfatin mRNA expression existed (both P >0.5). In conclusion, visfatin plasma concentrations and visceral visfatin mRNA expression correlated with measures of obesity but not with visceral fat mass or waist-to-hip ratio. In addition, we did not find differences in visfatin mRNA expression between visceral and subcutaneous adipose tissue in humans.
Berndt J, Klöting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54(10):2911-6.
How obesity causes diabetes: not a tall tale.
The epidemic of obesity-associated diabetes is a major crisis in modern societies, in which food is plentiful and exercise is optional. The biological basis of this problem has been explored from evolutionary and mechanistic perspectives. Evolutionary theories, focusing on the potential survival advantages of “thrifty” genes that are now maladaptive, are of great interest but are inherently speculative and difficult to prove. Mechanistic studies have revealed numerous fat-derived molecules and a link to inflammation that, together, are hypothesized to underlie the obesity-diabetes connection and thereby represent prospective targets for therapeutic intervention.
Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373-5.
Inflammation, stress, and diabetes.
Over the last decade, an abundance of evidence has emerged demonstrating a close link between metabolism and immunity. It is now clear that obesity is associated with a state of chronic low-level inflammation. In this article, we discuss the molecular and cellular underpinnings of obesity-induced inflammation and the signaling pathways at the intersection of metabolism and inflammation that contribute to diabetes. We also consider mechanisms through which the inflammatory response may be initiated and discuss the reasons for the inflammatory response in obesity. We put forth for consideration some hypotheses regarding important unanswered questions in the field and suggest a model for the integration of inflammatory and metabolic pathways in metabolic disease.
Tangvarasittichai S, Pingmuanglaew P, Tangvarasittichai O. Association of Elevated Serum Lipoprotein(a), Inflammation, Oxidative Stress and Chronic Kidney Disease with Hypertension in Non-diabetes Hypertensive Patients. Indian J Clin Biochem. 2016;31(4):446-51.
How does blood glucose control with insulin save lives in intensive care?
Patients requiring prolonged intensive care are at high risk for multiple organ failure and death. Insulin resistance and hyperglycemia accompany critical illness, and the severity of this “diabetes of stress” reflects the risk of death. Recently it was shown that preventing hyperglycemia with insulin substantially improves outcome of critical illness. This article examines some potential mechanisms underlying prevention of glucose toxicity as well as the effects of insulin independent of glucose control. Unraveling the molecular mechanisms will provide new insights into the pathogenesis of multiple organ failure and open avenues for novel therapeutic strategies.
Van den berghe G. How does blood glucose control with insulin save lives in intensive care?. J Clin Invest. 2004;114(9):1187-95
Turning down insulin signaling.
In recent years, much has been written about the importance of diabetes mellitus, both as a cause of widespread morbidity and mortality and in terms of the resultant overwhelming health care costs. In an analysis of worldwide diabetes in 1994, the World Health Organization reported that the age-standardized prevalence in European populations varied from 3% to 10%, while more restricted populations demonstrated prevalence of up to 50% (1). Over the same period, approximately 10.2 million people with diagnosed diabetes mellitus resided in the US, but another 5.4 million individuals with diabetes went undiagnosed (2). Perhaps even more impressive, these numbers have been estimated to represent as much as a 50% increase over the prevalence in equivalent populations during the previous decade. In 1997, health care expenditures attributable to diabetes in the US were estimated to be $98 billion (3). Undoubtedly, this is due not only to the widespread prevalence of the disease, but also to its chronic nature and disabling complications, affecting cardiovascular, renal, visual, and neurological function. Thus, perhaps the lack of an even more intense research effort into the pathophysiology of diabetes can only be attributed to the difficulty inherent in making progress in the study of this complex, multisystem disease. For these reasons, it is particularly important that in two recent articles — one appearing in the current issue of the journal Science and the other a recent issue of the JCI — Steven Shoelson, Gerald Shulman, and their colleagues present a new hypothesis, which not only purports to explain the insulin resistance of type 2 diabetes mellitus but also offers a clear basis for the development of novel therapeutics (4, 5).
Over 90% of diabetes mellitus is accounted for by what is now called the type 2 variant. Unlike type 1 diabetes, for which there is a reasonable consensus that the disease results from autoimmune destruction of insulin-secreting pancreatic β cells, the etiology of type 2 diabetes remains a bit uncertain. Most investigators and clinicians agree that genetic and environmental factors contribute and that obesity is a frequent if not essential antecedent of the disease. Perhaps the most heated debate among diabetes researchers has concerned the nature of the primary inciting metabolic event, that is, whether it represents a disturbance in the normal pattern of insulin secretion or abnormalities in the action of insulin in peripheral tissues (6). Experiments in which defects in insulin secretion or action have been selectively introduced into mice by the modification of single or multiple genes have been surprisingly unhelpful at resolving this issue. Perhaps these genetic studies only serve to emphasize the multi–organ system nature of diabetes mellitus, in which several defects are required to elicit sufficient dysfunction to overwhelm physiological compensatory mechanisms and produce diabetes.
Nevertheless, investigators have postulated a reasonable series of events to explain the evolution of type 2 diabetes (7). According to this model, peripheral insulin resistance represents the earliest event, but this is initially compensated by enhanced insulin secretion. Later, the β cell no longer keeps pace with the increased needs, and a relative lack of insulin is followed by an absolute deficiency of the hormone. At about the same time, the liver develops insulin resistance, thus leading to accelerated production of glucose. Whatever the precise sequence of the events by which impaired glucose tolerance matures to diabetes, there is little doubt that insulin resistance represents an important component of the fulminant disease.
Birnbaum MJ. Turning down insulin signaling. J Clin Invest. 2001;108(5):655-9.
Schematics