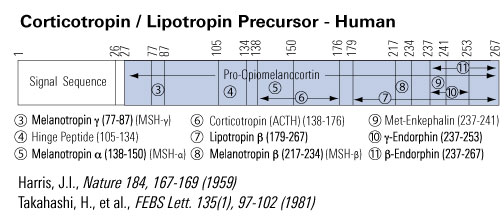
Melanocyte Stimulating Hormone
Abstract
Isolation and characterisation of a novel POMC-derived peptide from hemofiltrate of chronic renal failure patients.
We report the isolation of a novel human circulating proopiomelanocortin-derived peptide named VA-ß-MSH from hemofiltrate and its pharmacological characterization. Screening for lipolytic activity in differentiated 3T3-L1 adipocytes led to the isolation from a hemofiltrate peptide library by alternating reversed-phase and cation-exchange chromatography. In the course of this isolation, we also identified human ß-MSH (1-22). We synthesized VA-ß-MSH by the Fmoc (N-(9-fluorenyl)-methoxycarbonyl) solid phase method and used synthetic ß-MSH (1-22) to confirm that both isolated peptides are lipolytically active in a dosedependent manner in differentiated 3T3-L1 adipocytes in the nanomolar range. Using cAMPELISA, we demonstrate that stimulation with both peptides caused a strong cAMP elevation in this cell system. Furthermore, we show that the selective inhibitors of cAMP-dependent protein kinase, Rp-8-CPT-cAMPS and H89, significantly reduce VA-ß-MSH- and ß-MSH (1-22)-mediated lipolysis. Although isolated here following its lipolytic activity on 3T3-L1 cells, this newly identified circulating human melanocortin may also serve other functions in human physiology. Moreover, the fact that these peptides have been identified following a functional assay but have been overseen in large proteomic approaches underscore the importance for such approaches in order to identify previously undescribed circulating bioactive molecules.
Fricke K., et al. Endocrinology. First published January 13, 2005 as doi:10.1210/en.2004-1097
Harrold JA, et al. Peptides. 2003 Mar;24(3):397-405
In vivo multiplex quantitative analysis of 3 forms of α melanocyte stimulating hormone in pituitary of prolyl endopeptidase deficient mice.
CONCLUSION: The multiplex targeted quantitative peptidomics technique we present in this study will be decidedly useful to monitor several neuropeptide enzymatic reactions in vivo under varying conditions.
Perroud B, Alvarado RJ, Espinal GM, Morado AR, Phinney BS, Warden CH. Mol Brain. 2009;2:14.
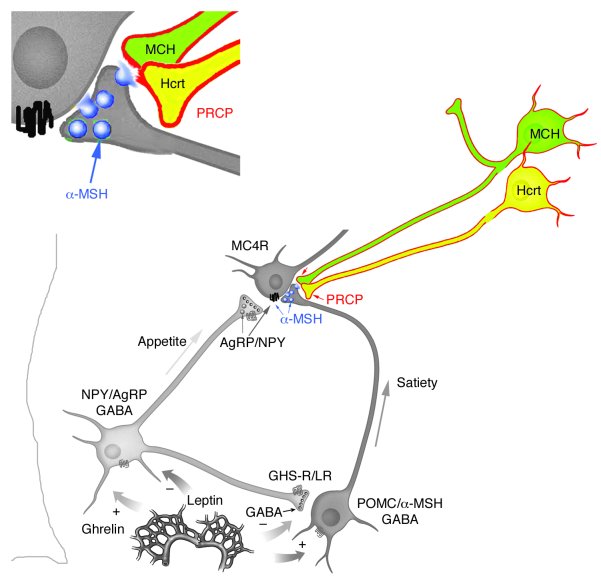
Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents.
The anorexigenic neuromodulator α-melanocyte-stimulating hormone (α-MSH; referred to here as α-MSH1-13) undergoes extensive posttranslational processing, and its in vivo activity is short lived due to rapid inactivation. The enzymatic control of α-MSH1-13 maturation and inactivation is incompletely understood. Here we have provided insight into α-MSH1-13 inactivation through the generation and analysis of a subcongenic mouse strain with reduced body fat compared with controls. Using positional cloning, we identified a maximum of 6 coding genes, including that encoding prolylcarboxypeptidase (PRCP), in the donor region. Real-time PCR revealed a marked genotype effect on Prcp mRNA expression in brain tissue. Biochemical studies using recombinant PRCP demonstrated that PRCP removes the C-terminal amino acid of α-MSH1-13, producing α-MSH1-12, which is not neuroactive. We found that Prcp was expressed in the hypothalamus in neuronal populations that send efferents to areas where α-MSH1-13 is released from axon terminals. The inhibition of PRCP activity by small molecule protease inhibitors administered peripherally or centrally decreased food intake in both wild-type and obese mice. Furthermore, Prcp-null mice had elevated levels of α-MSH1-13 in the hypothalamus and were leaner and shorter than the wild-type controls on a regular chow diet; they were also resistant to high-fat diet-induced obesity. Our results suggest that PRCP is an important component of melanocortin signaling and weight maintenance via control of active α-MSH1-13 levels.
Wallingford N, Perroud B, Gao Q, et al. J Clin Invest. 2009;119(8):2291-303.
Isolation and characterisation of a novel POMC-derived peptide from hemofiltrate of chronic renal failure patients.
Fricke K., et al. Endocrinology. First published January 13, 2005 as doi:10.1210/en.2004-1097
γ-MSH increases intracellular cAMP accumulation and GnRH release in vitro and LH release in vivo
Stanley SA, et al. FEBS Lett. 2003 May 22;543(1-3):66-70
Schematics

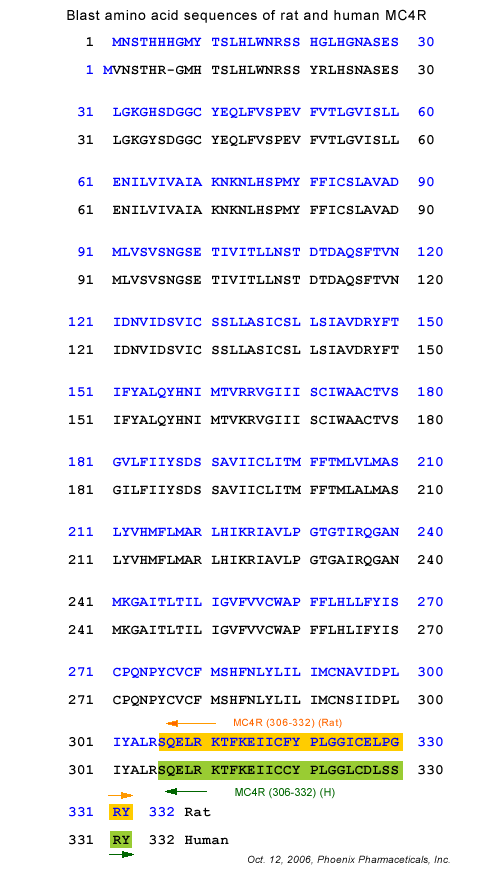
More Information
Hormone Overview
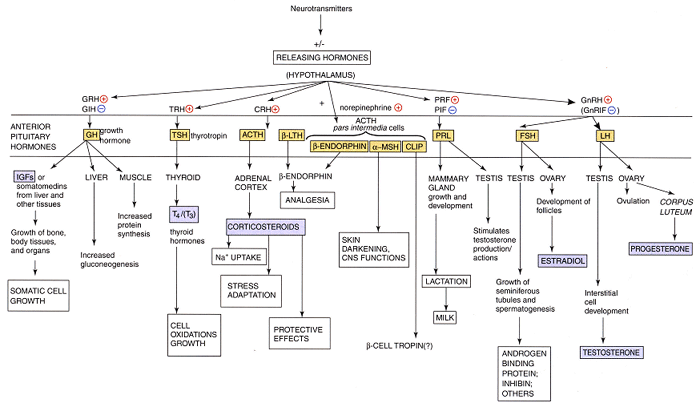
Role of Portal System
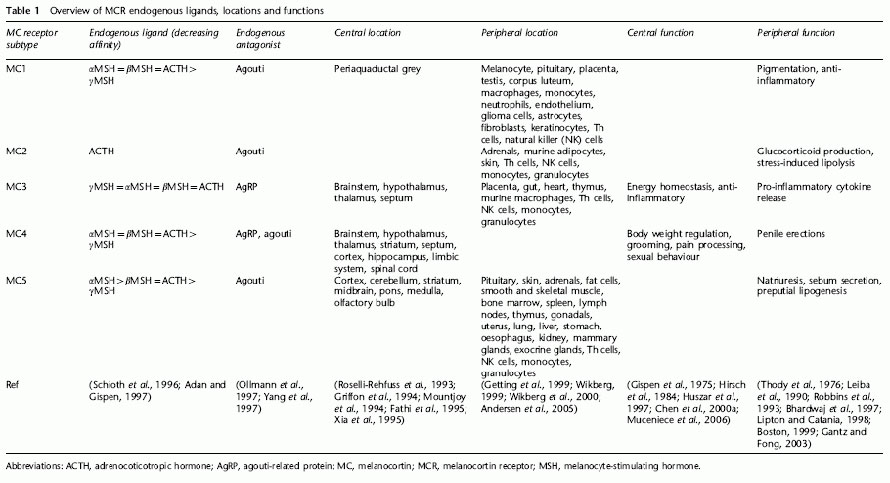
Overview of MCR endogenous ligands, locations and functions
from: R A H Adan et al. The MC4 receptor and control of appetite. British Journal of Pharmacology (2006) 149, 815–827