Exendin-4 (Heloderma suspectum) – EIA Kit, CE Mark Certified
- Product Category: ELISA Kits - Assay Kits - RIA Kits, ELISA-EIA
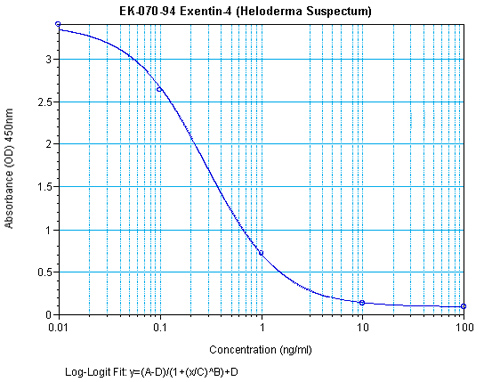
Catalog #: EK-070-94CE
Size: 96 wells
Price: $632
Intra-assay variation: <10%
Inter-assay variation: <15%
| Peptide | % |
| Exendin-4 (Heloderma Suspectum) | 100 |
| Exendin-3 (9-39)-NH2 | 100 |
| Exendin-4 (3-39) | 100 |
| Lixisenatide | 100 |
| Glucagon (Human, Rat, Mouse, Porcine, Bovine) | 0 |
| GLP-1 (7-36)-NH2 (Human, Rat, Mouse) | 0 |
| GLP-1 (7-37) (Human, Rat, Mouse) | 0 |
| GLP-2 (Human) | 0 |
| Oxyntomodulin (Human, Rat, Mouse) | 0 |
No results found.
Links to publications that use this kit:
C-terminal site-specific PEGylated Exendin-4 analog: A long-acting glucagon like Peptide-1 receptor agonist, on glycemic control and beta cell function in diabetic db/db mice.
Tang D, Tian H, Wu J, et al. J Pharmacol Sci. 2018;138(1):23-30.
GLP-1 receptor agonists stimulate ANGPTL8 production through the PI3K/Akt pathway in a GLP-1 receptor-dependent manner.
Liu J, Yang K, Xiao W, et al. Peptides. 2018;106:83-90.
The use of low molecular weight protamine to enhance oral absorption of exenatide.
Zhang L, Shi Y, Song Y, et al. Int J Pharm. 2018;547(1-2):265-273.
Replacement of the C-terminal Trp-cage of exendin-4 with a fatty acid improves therapeutic utility.
Lee JG, Ryu JH, Kim SM, et al. Biochem Pharmacol. 2018;151:59-68.
Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: in vitro characterization and in vivo evaluation.
Chen C, Zheng H, Xu J, Shi X, Li F, Wang X. Daru. 2017;25(1):20.
In vitro and in vivo characterization of a novel long-acting GLP-1 receptor agonist, exendin-4–Fc fusion protein
Lian Lu, Xiaoqing Su, Yantai Wang et al., RSC Adv., 2017,7, 54178-54187
An approach for half-life extension and activity preservation of an anti-diabetic peptide drug based on genetic fusion with an albumin-binding aptide.
Kim D, Jeon H, Ahn S, Choi WI, Kim S, Jon S. J Control Release. 2017;256:114-120.
Pyung-Hwan Kim, Minhyung Lee, Sung Wan Kim, Delivery of two-step transcription amplification exendin-4 plasmid system with arginine-grafted bioreducible polymer in type 2 diabetes animal model.
J Control Release. 2012 Aug 20;162(1):9-18. doi: 10.1016/j.jconrel.2012.06.010. Epub 2012 Jun 15.
PLoS ONE. 2012;7(7):e40074.
Nian Gong et al., Site-specific PEGylation of exenatide analogues markedly improved their glucoregulatory activity
Br J Pharmacol. 2011 May; 163(2): 399–412.
Wei Gao et al., Pharmacokinetic and Pharmacodynamic Modeling of Exendin-4 in Type 2 Diabetic Goto-Kakizaki Rats
J Pharmacol Exp Ther. 2011 March; 336(3): 881–890. doi: 10.1124/jpet.110.175752
Su Young Chaea, Cheng-Hao Jin et al., Biochemical, pharmaceutical and therapeutic properties of long-acting lithocholic acid derivatized exendin-4 analogs
Journal of Controlled Release Volume 142, Issue 2, 3 March 2010, Pages 206–213
Kamei et al. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides.
J Control Release. 2009 Feb 27. [Epub ahead of print]
Samson et al. Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model.
Mol Ther. 2008 Nov;16(11):1805-12.