LGR7 (Relaxin Receptor 1) and LGR8 (Relaxin Receptor 2), receptors for Insulin-like peptide 3 & 7
Abstract
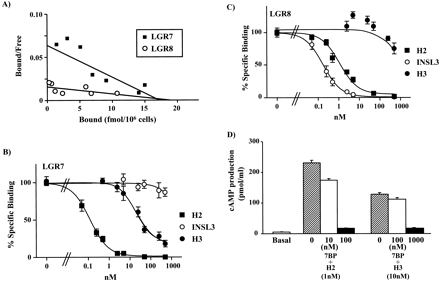
H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2.
Leucine-rich repeat-containing, G protein-coupled receptors (LGRs) represent a unique subgroup of G protein-coupled receptors with a large ectodomain. Recent studies demonstrated that relaxin activates two orphan LGRs, LGR7 and LGR8, whereas INSL3/Leydig insulin-like peptide specifically activates LGR8. Human relaxin 3 (H3 relaxin) was recently discovered as a novel ligand for relaxin receptors. Here, we demonstrate that H3 relaxin activates LGR7 but not LGR8. Taking advantage of the overlapping specificity of these three ligands for the two related LGRs, chimeric receptors were generated to elucidate the mechanism of ligand activation of LGR7. Chimeric receptor LGR7/8 with the ectodomain from LGR7 but the transmembrane region from LGR8 maintains responsiveness to relaxin but was less responsive to H3 relaxin based on ligand stimulation of cAMP production. The decreased ligand signaling was accompanied by decreases in the ability of H3 relaxin to compete for (33)P-relaxin binding to the chimeric receptor. However, replacement of the exoloop 2, but not exoloop 1 or 3, of LGR7 to the chimeric LGR7/8 restored ligand binding and receptor-mediated cAMP production. These results suggested that activation of LGR7 by H3 relaxin involves specific binding of the ligand to both the ectodomain and the exoloop 2, thus providing a model with which to understand the molecular basis of ligand signaling for this unique subgroup of G protein-coupled receptors.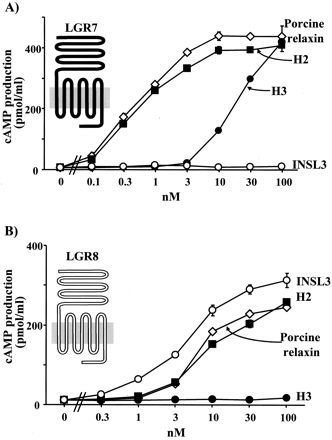
Sudo S, Kumagai J, Nishi S, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem. 2003;278(10):7855-62.
GREAT/LGR8 is the only receptor for insulin-like 3 peptide.
During male development testes descend from their embryonic intraabdominal position into the scrotum. Two genes, encoding the insulin-like 3 peptide (INSL3) and the GREAT/LGR8 G protein-coupled receptor, control the differentiation of gubernaculum, the caudal genitoinguinal ligament critical for testicular descent. It was established that the INSL3 peptide activates GREAT/LGR8 receptor in vitro. Mutations of Insl3 or Great cause cryptorchidism (undescended testes) in mice. Overexpression of the transgenic Insl3 causes male-like gubernaculum differentiation, ovarian descent into lower abdominal position, and reduced fertility in females. To address the question whether Great deletion complements the mutant female phenotype caused by the Insl3 overexpression, we have produced Insl3 transgenic mice deficient for Great. Such females had a wild-type phenotype, demonstrating that Great was the only cognate receptor for Insl3 in vivo. We have established that pancreatic HIT cells, transfected with the INSL3 cDNA, produce functionally active peptide. Analysis of five INSL3 mutant variants detected in cryptorchid patients showed that P49S substitution renders functionally compromised peptide. Therefore, mutations in INSL3 might contribute to the etiology of cryptorchidism. We have also showed that synthetic insulin-like peptides (INSL4 and INSL6) were unable to activate LGR7 or GREAT/LGR8.
Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol. 2003;17(12):2639-46.
Activation of orphan receptors by the hormone relaxin.
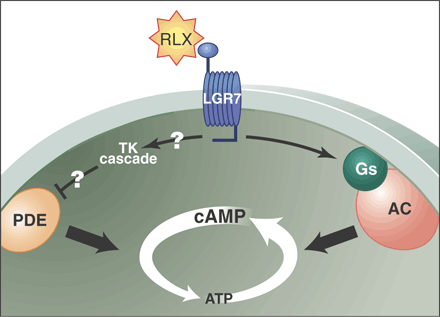
Relaxin is a hormone important for the growth and remodeling of reproductive and other tissues during pregnancy. Although binding sites for relaxin are widely distributed, the nature of its receptor has been elusive. Here, we demonstrate that two orphan heterotrimeric guanine nucleotide binding protein (G protein)-coupled receptors, LGR7 and LGR8, are capable of mediating the action of relaxin through an adenosine 3′,5′-monophosphate (cAMP)-dependent pathway distinct from that of the structurally related insulin and insulin-like growth factor family ligand. Treatment of antepartum mice with the soluble ligand-binding region of LGR7 caused parturition delay. The wide and divergent distribution of the two relaxin receptors implicates their roles in reproductive, brain, renal, cardiovascular, and other functions.
Hsu SY, Nakabayashi K, Nishi S, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295(5555):671-4.
Schematics

More Information