Neuropeptide Y
Abstract
Park MH, Baek B, Jin HK, Bae JS. Novel peptides derived from neuropeptide Y prevent chemotherapy-induced bone marrow damage by regulating hematopoietic stem cell microenvironment. Anim Cells Syst (Seoul). 2018;22(5):281-288.
Wagner L, Björkqvist M, Lundh SH, et al. Neuropeptide Y (NPY) in cerebrospinal fluid from patients with Huntington’s Disease: increased NPY levels and differential degradation of the NPY1-30 fragment. J Neurochem. 2016;137(5):820-37.
Rose JB, Crews L, Rockenstein E, et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer’s disease. J Neurosci. 2009;29(4):1115-25.
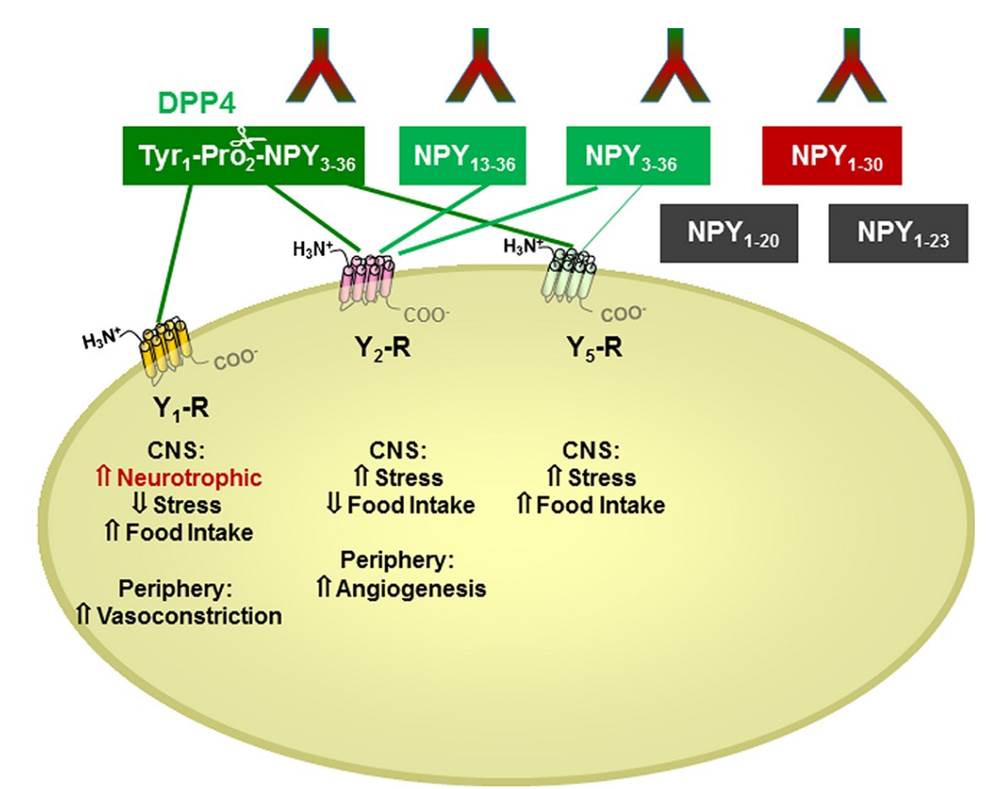
Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35, a new peptide cleaved by plasma kallikrein.
There is little information on how neuropeptide Y (NPY) proteolysis by peptidases occurs in serum, in part because reliable techniques are lacking to distinguish different NPY immunoreactive forms and also because the factors affecting the expression of these enzymes have been poorly studied. In the present study, LC-MS/MS was used to identify and quantify NPY fragments resulting from peptidolytic cleavage of NPY1–36 upon incubation with human serum. Kinetic studies indicated that NPY1–36 is rapidly cleaved in serum into 3 main fragments with the following order of efficacy: NPY3–36 ? NPY3–35 > NPY2–36. Trace amounts of additional NPY forms were identified by accurate mass spectrometry. Specific inhibitors of dipeptidyl peptidase IV, kallikrein, and aminopeptidase P prevented the production of NPY3–36, NPY3–35, and NPY2–36, respectively. Plasma kallikrein at physiological concentrations converted NPY3–36 into NPY3–35. Receptor binding assays revealed that NPY3–35 is unable to bind to NPY Y1, Y2, and Y5 receptors; thus NPY3–35 may represent the major metabolic clearance product of the Y2/Y5 agonist, NPY3–36.
Abid K, Rochat B, Lassahn PG, et al. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35: a new peptide generated by plasma kallikrein. J Biol Chem. 2009;284(37):24715-24
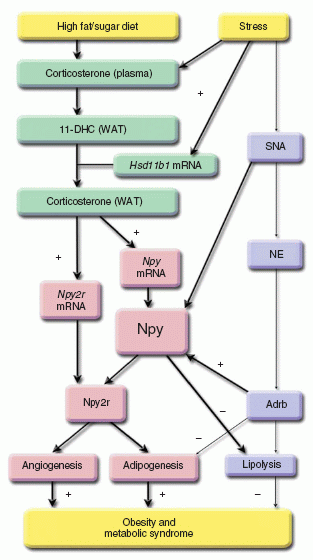
Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome.
The relationship between stress and obesity remains elusive. In response to stress, some people lose weight, whereas others gain. Here we report that stress exaggerates diet-induced obesity through a peripheral mechanism in the abdominal white adipose tissue that is mediated by neuropeptide Y (NPY). Stressors such as exposure to cold or aggression lead to the release of NPY from sympathetic nerves, which in turn upregulates NPY and its Y2 receptors (NPY2R) in a glucocorticoid-dependent manner in the abdominal fat. This positive feedback response by NPY leads to the growth of abdominal fat. Release of NPY and activation of NPY2R stimulates fat angiogenesis, macrophage infiltration, and the proliferation and differentiation of new adipocytes, resulting in abdominal obesity and a metabolic syndrome-like condition. NPY, like stress, stimulates mouse and human fat growth, whereas pharmacological inhibition or fat-targeted knockdown of NPY2R is anti-angiogenic and anti-adipogenic, while reducing abdominal obesity and metabolic abnormalities. Thus, manipulations of NPY2R activity within fat tissue offer new ways to remodel fat and treat obesity and metabolic syndrome.
Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803-11
The first selective agonist for the neuropeptide Y Y5-receptor increases food intake in rats.
The first Y(5) receptor-selective analog of neuropeptide Y (NPY), [Ala(31),Aib(32)]NPY, has been developed and biologically characterized. Using competition binding assays on cell lines that express different Y receptors, we determined the affinity of this analog to be 6 nm at the human Y(5) receptor, >500 nm at the Y(1) and Y(2) receptors, and >1000 nm at the Y(4) receptor. Activity studies performed in vitro using a cAMP enzyme immunoassay, and in vivo using food intake studies in rats, showed that the peptide acted as an agonist. Further peptides obtained by the combination of the Ala(31)-Aib(32) motif with chimeric peptides containing segments of NPY and pancreatic polypeptide displayed the same selectivity and even higher affinity (up to 0.2 nm) for the Y(5) receptor. In vivo administration of the new Y(5) receptor-selective agonists significantly stimulated feeding in rats. The NMR solution structures of NPY and [Ala(31),Aib(32)]NPY showed a different conformation in the C-terminal region, where the alpha-helix of NPY was substituted by a more flexible, 3(10)-helical turn structure.
Cabrele C, Langer M, Bader R, et al. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem. 2000;275(46):36043-8
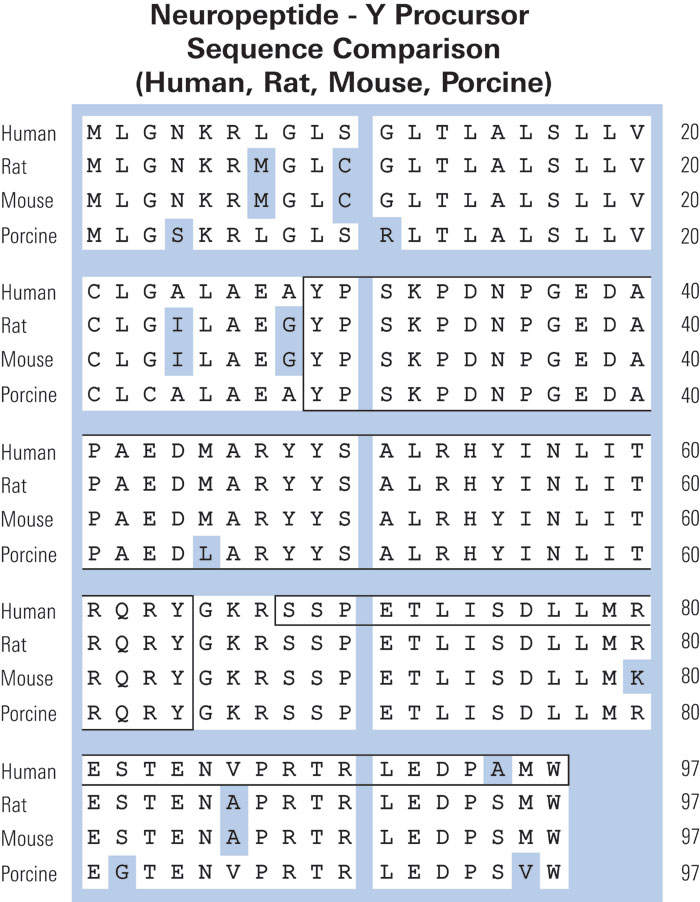
Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers.
Immunoreactive-neuropeptide Y (i-NPY) is present in platelets of rats, and has recently been demonstrated to be authentic rat NPY based on its amino acid sequence. This potent vasoconstrictor and putative smooth muscle mitogen is released during platelet activation, suggesting a role in platelet-vascular interactions. We have now extended this work to several strains of rats and mice, and humans of both sexes. Among mice, strains in which NPY mRNA has been demonstrated in megakaryocytes have markedly higher levels of i-NPY (0.63-1.11 pmol/ml in NZB/B1NJ, NZBWF1/J, BXSB/MpJYaa, BALB/cJ) in platelet rich plasma (PRP) than other strains (DBA/2J, CBA/J, C3H/HeJ, MRL/MpJ-lpr, C57BL/6J; each < 0.02 pmol/ml). In rats, high content of i-NPY was observed in PRP and platelets of all strains examined (Sprague-Dawley, Wistar, Wistar Kyoto). i-NPY level was 30.6, 3.7 and 10.1 pmol/ml in PRP of the three strains, respectively. In humans, low levels of i-NPY occur in plasma and platelet fractions compared to rodents (0.069 and 0.048 pmol/ml in male and female PRP, respectively), but they, too, have greater i-NPY in platelet rich plasma and platelets than in platelet poor plasma. Assuming this is authentic NPY, platelet-derived NPY might have a role in pathophysiological states involving activation of platelets in humans.
Myers AK, Torres duarte AP, Zukowska-grojec Z. Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers. Regul Pept. 1993;47(3):239-4
Schematics
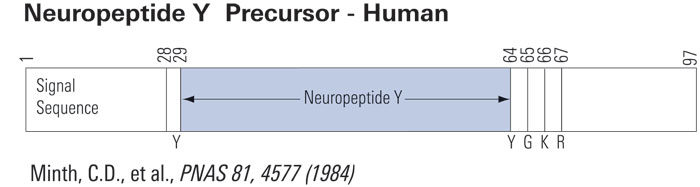
 |
|
More Information