Cancer-Targeting Peptide Library
Abstract
Antimicrobial peptides (AMPs) are a pervasive and evolutionarily ancient component of innate host defense which is present in virtually all classes of life. In recent years, evidence has accumulated that parallel or de novo mechanisms by which AMPs curb infectious pathologies are also effective at restraining cancer cell proliferation and dissemination, and have consequently stimulated significant interest in their deployment as novel biologic and immunotherapeutic agents against human malignancies. In this review, we explicate the biochemical underpinnings of their tumor-selectivity, and discuss results of recent clinical trials (outside of oncologic indications) which substantiate their safety and tolerability profiles. Next, we present evidence for their preclinical antitumor activity, systematically organized by the major and minor classes of natural AMPs. Finally, we discuss the barriers to their clinical implementation and envision directions for further development.
Roudi R, Syn NL, Roudbary M. Antimicrobial Peptides As Biologic and Immunotherapeutic Agents against Cancer: A Comprehensive Overview. Front Immunol. 2017;8:1320.
Tumor-targeted delivery of siRNA using fatty acyl-CGKRK peptide conjugates.
Tumor-targeted carriers provide efficient delivery of chemotherapeutic agents to tumor tissue. CGKRK is one of the well-known tumor targeting peptides with significant specificity for angiogenic blood vessels and tumor cells. Here, we designed fatty acyl conjugated CGKRK peptides, based on the hypothesis that hydrophobically-modified CGKRK peptide could enhance cellular permeation and delivery of siRNA targeted to tumor cells for effective silencing of chosen proteins. We synthesized six fatty acyl-peptide conjugates, using a diverse chain of saturated and unsaturated fatty acids to study the efficiency of this approach. At peptide: siRNA weight/weight ratio of 10:1 (N/P ≈ 13.6), almost all the peptides showed complete binding with siRNA, and at a w/w ratio of 20:1 (N/P ≈ 27.3), complete protection of siRNA from early enzymatic degradation was observed. Conjugated peptides and peptide/siRNA complexes did not show significant cytotoxicity in certain cell lines. The oleic acid-conjugated peptide showed the highest efficiency in siRNA uptake and silencing of kinesin spindle protein at peptide:siRNA w/w ratio of 80:1 (N/P ≈ 109). The siRNA internalization into non-tumorigenic kidney cells was negligible with all fatty acyl-peptide conjugates. These results indicate that conjugation of fatty acids to CGKRK could create an efficient delivery system for siRNA silencing specifically in tumor cells.
Sharma M, El-sayed NS, Do H, Parang K, Tiwari RK, Aliabadi HM. Sci Rep. 2017;7(1):6093.
Tumor-homing peptides as tools for targeted delivery of payloads to the placenta.
The availability of therapeutics to treat pregnancy complications is severely lacking mainly because of the risk of causing harm to the fetus. As enhancement of placental growth and function can alleviate maternal symptoms and improve fetal growth in animal models, we have developed a method for targeted delivery of payloads to the placenta. We show that the tumor-homing peptide sequences CGKRK and iRGD bind to the placental surface of humans and mice and do not interfere with normal development. Peptide-coated nanoparticles intravenously injected into pregnant mice accumulated within the mouse placenta, whereas control nanoparticles exhibited reduced binding and/or fetal transfer. We used targeted liposomes to efficiently deliver cargoes of carboxyfluorescein and insulin-like growth factor 2 to the mouse placenta; the latter significantly increased mean placental weight when administered to healthy animals and significantly improved fetal weight distribution in a well-characterized model of fetal growth restriction. These data provide proof of principle for targeted delivery of drugs to the placenta and provide a novel platform for the development of placenta-specific therapeutics.
King A, Ndifon C, Lui S, et al. Sci Adv. 2016;2(5):e1600349.
Tumor-penetrating iRGD peptide inhibits metastasis.
Tumor-specific tissue-penetrating peptides deliver drugs into extravascular tumor tissue by increasing tumor vascular permeability through interaction with neuropilin (NRP). Here, we report that a prototypic tumor-penetrating peptide iRGD (amino acid sequence: CRGDKGPDC) potently inhibits spontaneous metastasis in mice. The antimetastatic effect was mediated by the NRP-binding RXXK peptide motif (CendR motif), and not by the integrin-binding RGD motif. iRGD inhibited migration of tumor cells and caused chemorepulsion in vitro in a CendR- and NRP-1-dependent manner. The peptide induced dramatic collapse of cellular processes and partial cell detachment, resulting in the repellent activity. These effects were prominently displayed when the cells were seeded on fibronectin, suggesting a role of CendR in functional regulation of integrins. The antimetastatic activity of iRGD may provide a significant additional benefit when this peptide is used for drug delivery to tumors.
Sugahara KN, Braun GB, De mendoza TH, et al. Mol Cancer Ther. 2015;14(1):120-8.
Tumor-penetrating peptides.
Tumor-homing peptides can be used to deliver drugs into tumors. Phage library screening in live mice has recently identified homing peptides that specifically recognize the endothelium of tumor vessels, extravasate, and penetrate deep into the extravascular tumor tissue. The prototypic peptide of this class, iRGD (CRGDKGPDC), contains the integrin-binding RGD motif. RGD mediates tumor-homing through binding to ?v integrins, which are expressed on various cells in tumors, including tumor endothelial cells. The tumor-penetrating properties of iRGD are mediated by a second sequence motif, R/KXXR/K. This C-end Rule (or CendR) motif is active only when the second basic residue is exposed at the C-terminus of the peptide. Proteolytic processing of iRGD in tumors activates the cryptic CendR motif, which then binds to neuropilin-1 activating an endocytic bulk transport pathway through tumor tissue. Phage screening has also yielded tumor-penetrating peptides that function like iRGD in activating the CendR pathway, but bind to a different primary receptor. Moreover, novel tumor-homing peptides can be constructed from tumor-homing motifs, CendR elements and protease cleavage sites. Pathologies other than tumors can be targeted with tissue-penetrating peptides, and the primary receptor can also be a vascular “zip code” of a normal tissue. The CendR technology provides a solution to a major problem in tumor therapy, poor penetration of drugs into tumors. The tumor-penetrating peptides are capable of taking a payload deep into tumor tissue in mice, and they also penetrate into human tumors ex vivo. Targeting with these peptides specifically increases the accumulation in tumors of a variety of drugs and contrast agents, such as doxorubicin, antibodies, and nanoparticle-based compounds. Remarkably the drug to be targeted does not have to be coupled to the peptide; the bulk transport system activated by the peptide sweeps along any compound that is present in the blood.
Teesalu T, Sugahara KN, Ruoslahti E. Front Oncol. 2013;3:216.
NGR-peptide-drug conjugates with dual targeting properties.
Peptides containing the asparagine-glycine-arginine (NGR) motif are recognized by CD13/aminopeptidase N (APN) receptor isoforms that are overexpressed in tumor neovasculature. Spontaneous decomposition of NGR peptides can result in isoAsp derivatives, which are recognized by RGD-binding integrins that are essential for tumor metastasis. Peptides binding to CD13 and RGD-binding integrins provide tumor-homing, which can be exploited for dual targeted delivery of anticancer drugs. We synthesized small cyclic NGR peptide-daunomycin conjugates using NGR peptides of varying stability (c[KNGRE]-NH2, Ac-c[CNGRC]-NH2 and the thioether bond containing c[CH2-CO-NGRC]-NH2, c[CH2-CO-KNGRC]-NH2). The cytotoxic effect of the novel cyclic NGR peptide-Dau conjugates were examined in vitro on CD13 positive HT-1080 (human fibrosarcoma) and CD13 negative HT-29 (human colon adenocarcinoma) cell lines. Our results confirm the influence of structure on the antitumor activity and dual acting properties of the conjugates. Attachment of the drug through an enzyme-labile spacer to the C-terminus of cyclic NGR peptide resulted in higher antitumor activity on both CD13 positive and negative cells as compared to the branching versions.
Andrei Timotin, Oleg Pisarenko, Maria Sidorova et al., Oncotarget. 2017 Mar 28; 8(13): 21241–21252.
iRGD as a tumor-penetrating peptide for cancer therapy (Review).
As a tumor-targeting and ?penetrating peptide, iRGD binds to αv integrins and neuropilin-1 receptors, which are expressed at high levels on tumor cells and the surfaces of vasculature. Subsequently, iRGD penetrates deep into the tumor parenchyma with antitumor drugs, imaging agents, immune modulators and biological products. These substances are either chemically linked to the peptide or co?injected with the peptide. The iRGD peptide can be readily synthesized, exhibits significantly improved penetration, compared with traditional peptides, and can effectively inhibit tumor metastasis. Therefore, the peptide is now used widely for the diagnosis and treatment of cancer. However, whether the peptide is able to promote the entry of drugs into non-targeted cells remains to be fully elucidated. In this review, an overview of iRGD is presented, focusing on its identification, mechanism of action and previous studies on its roles in various types of cancer. Studies in previous years have demonstrated the potential of the iRGD protein for tumors diagnosis and targeted treatment, which warrants further investigation.
Yin H, Yang J, Zhang Q, et al. Mol Med Rep. 2017;15(5):2925-2930.
BACKGROUND: Cancer is responsible for millions of immature deaths every year and is an economical burden on developing countries. One of the major challenges in the present era is to design drugs that can specifically target tumor cells not normal cells. In this context, tumor homing peptides have drawn much attention. These peptides are playing a vital role in delivering drugs in tumor tissues with high specificity. In order to provide service to scientific community, we have developed a database of tumor homing peptides called TumorHoPe.
DESCRIPTION: TumorHoPe is a manually curated database of experimentally validated tumor homing peptides that specifically recognize tumor cells and tumor associated microenvironment, i.e., angiogenesis. These peptides were collected and compiled from published papers, patents and databases. Current release of TumorHoPe contains 744 peptides. Each entry provides comprehensive information of a peptidethat includes its sequence, target tumor, target cell, techniques of identification, peptide receptor, etc. In addition, we have derived various types of information from these peptide sequences that include secondary/tertiary structure, amino acid composition, and physicochemical properties of peptides. Peptides in this database have been found to target different types of tumors that include breast, lung, prostate, melanoma, colon, etc. These peptides have some common motifs including RGD (Arg-Gly-Asp) and NGR (Asn-Gly-Arg) motifs, which specifically recognize tumor angiogenic markers. TumorHoPe has been integrated with many web-based tools like simple/complex search, database browsing and peptide mapping. These tools allow a user to search tumor homing peptides based on their amino acid composition, charge, polarity, hydrophobicity, etc.
CONCLUSION: TumorHoPe is a unique database of its kind, which provides comprehensive information about experimentally validated tumor homing peptides and their target cells. This database will be very useful in designing peptide-based drugs and drug-delivery system. It is freely available at
Kapoor P, Singh H, Gautam A, Chaudhary K, Kumar R, Raghava GP. PLoS ONE. 2012;7(4):e35187.
More Information
The Cancer-Targeting Peptide Library from Phoenix Pharmaceuticals contains a specially curated collection of peptides with cancer promoting or anti-cancer propreties. Each peptide was selected based on published articles, patents, and internal data supporting their role in the cancer pathway. Generally, they can be separated into the following three categories.
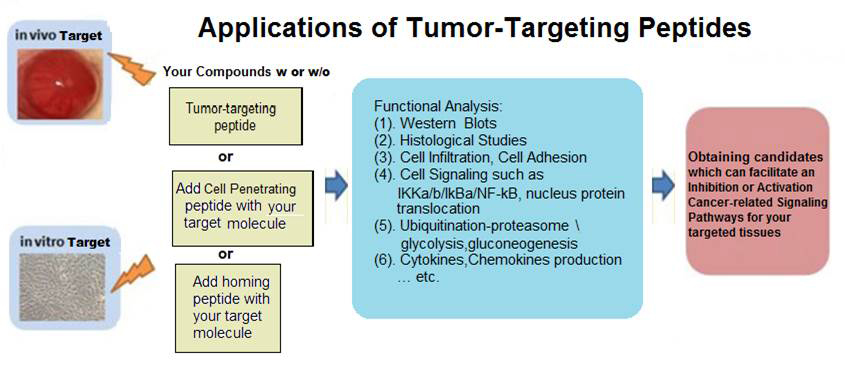
Effects of antimicrobial peptides on in preclinical models of neoplasia.
Several recurrent themes have emerged from unfolding research on their anticancer properties, including their antiproliferative and antimetastatic capabilities, invigoration of antitumor immunity, activity against multidrug-resistant cancer cells, and selectivity for cancer cells but not normal cells.