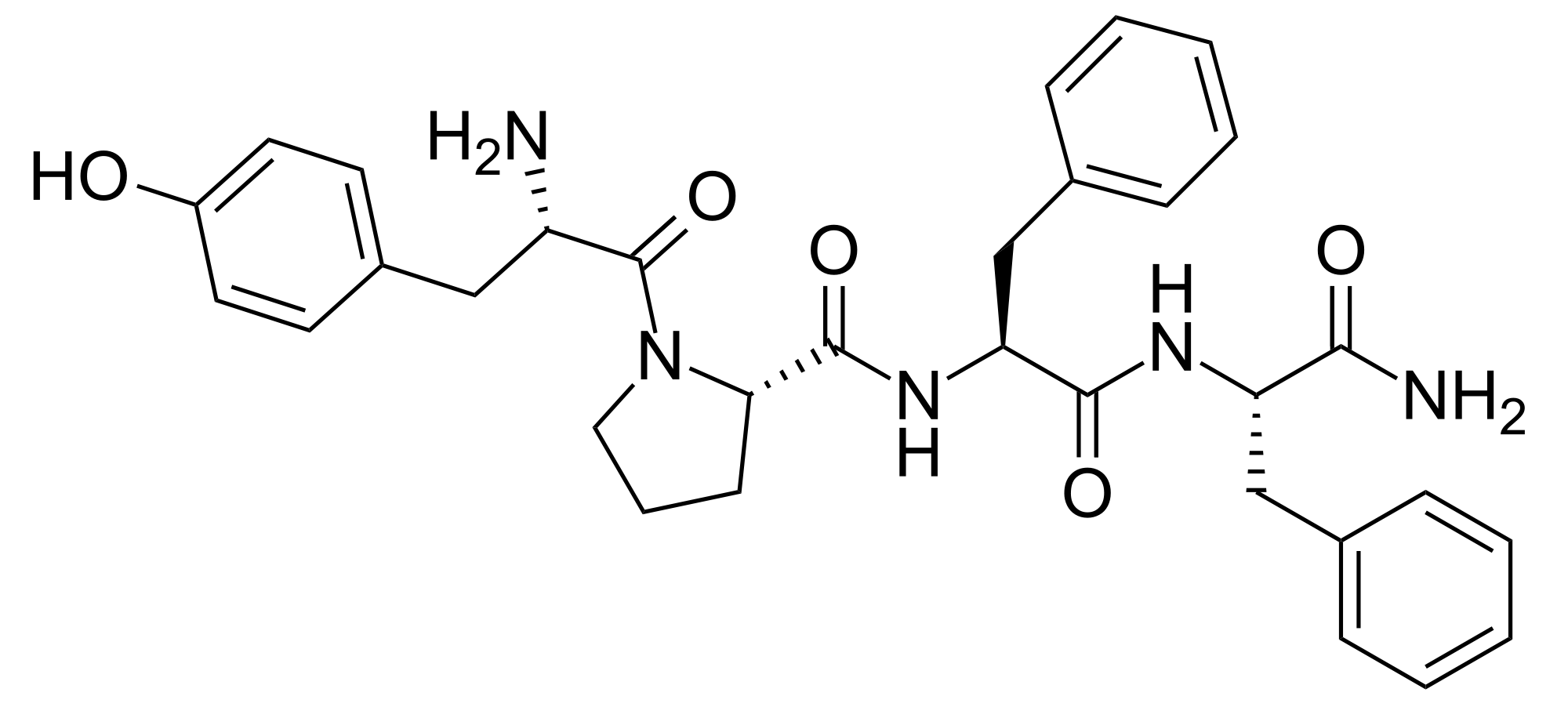
Endomorphins and related opioid peptides
Abstract
Endomorphins and related opioid peptides.
Opioid peptides and their G-protein-coupled receptors (delta, kappa, mu) are located in the central nervous system and peripheral tissues. The opioid system has been studied to determine the intrinsic mechanism of modulation of pain and to develop uniquely effective pain-control substances with minimal abuse potential and side effects. Two types of endogenous opioid peptides exist, one containing Try-Gly-Gly-Phe as the message domain (enkephalins, endorphins, dynorphins) and the other containing the Tyr-Pro-Phe/Trp sequence (endomorphins-1 and -2). Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2), which has high mu receptor affinity (Ki = 0.36 nM) and remarkable selectivity (4000- and 15,000-fold preference over the delta and kappa receptors, respectively), was isolated from bovine and human brain. In addition, endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), isolated from the same sources, exhibited high mu receptor affinity (Ki = 0.69 nM) and very high selectivity (13,000- and 7500-fold preference relative to delta and kappa receptors, respectively). Both opioids bind to mu-opioid receptors, thereby activating G-proteins, resulting in regulation of gastrointestinal motility, manifestation of antinociception, and effects on the vascular systems and memory. To develop novel analgesics with less addictive properties, evaluation of the structure-activity relationships of the endomorphins led to the design of more potent and stable analgesics. Opioidmimetics and opioid peptides containing the amino acid sequence of the message domain of endomorphins, Tyr-Pro-Phe/Trp, could exhibit unique binding activity and lead to the development of new therapeutic drugs for controlling pain.
Okada Y, Tsuda Y, Bryant SD, Lazarus LH. Endomorphins and related opioid peptides. Vitam Horm. 2002;65:257-79.
Receptor constants for endomorphin-1 and endomorphin-1-ol indicate differences in efficacy and receptor occupancy.
The opioid properties of endomorphin derivatives containing a C-terminal alcoholic(-ol) function were compared to the parent amidated compounds in isolated organs (longitudinal muscle strip of guinea-pig ileum and mouse vas deferens). Similar data were also generated for the mu-opioid receptor selective agonist synthetic peptide (D-Ala2, MePhe4, Gly5-ol)-enkephalin (DAMGO) and its Gly5-NH2 congener (DAMGA). Endomorphin-1-ol (Tyr-Pro-Trp-Phe-ol) had an IC50 of 80.6 nM in mouse vas deferens and 61.2 nM in guinea-pig ileum; the corresponding values for endomorphin-2-ol (Tyr-Pro-Phe-Phe-ol) were 49.6 and 48.2 nM, for DAMGO 59.8 and 29.2 nM, respectively. As it was indicated by the antagonism by naltrexone, the agonist actions were exerted exclusively at mu-opioid receptors in both organs. The -ol derivatives were slightly (2.3-4.3 times) less potent than the parent amides in the bioassays: all peptides had, apparently, full agonist properties in intact preparations. With the aim of revealing potential partial agonist properties among the investigated peptides, we partially inactivated the mu-opioid receptor pool in mouse vas deferens by 5×10(-7) M beta-funaltrexamine. The calculated receptor constants indicated a “high-affinity, low intrinsic efficacy” profile (i.e. a potential partial agonist property) for endomorphin-1, an intermediate character for endomorpin-1-ol and full agonism for DAMGA and DAMGO. Apparently, a higher receptor fraction remained accessible for endomorphin-1 (42.8%) than for the -ol congener (14.0%), DAMGO (20.2%) and DAMGA (14.1%) after partial inactivation.
Al-khrasani M, Orosz G, Kocsis L, et al. Receptor constants for endomorphin-1 and endomorphin-1-ol indicate differences in efficacy and receptor occupancy. Eur J Pharmacol. 2001;421(1):61-7.
Specific activation of the mu opioid receptor (MOR) by endomorphin 1 and endomorphin.
The recently discovered endomorphin 1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin 2 (Tyr-Pro-Phe-Phe-NH2) were investigated with respect to their direct receptor-binding properties, and to their ability to activate G proteins and to inhibit adenylyl cyclase in both cellular and animal models. Both tetrapeptides activated G proteins and inhibited adenylyl cyclase activity in membrane preparations from cells stably expressing the mu opioid receptor, an effect reversed by the mu receptor antagonist CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2), but they had no influence on cells stably expressing the delta opioid receptor. To further establish the selectivity of these peptides for the mu opioid receptor, brain preparations of mice lacking the mu opioid receptor gene were used to study their binding and signalling properties. Endomorphin 2, tritiated by a dehalotritiation method resulting in a specific radioactivity of 1.98 TBq/mmol (53.4 Ci/mmol), labelled the brain membranes of wild-type mice with a Kd value of 1.77 nM and a Bmax of 63.33 fmol/mg protein. In membranes of mice lacking the mu receptor gene, no binding was observed, and both endomorphins failed to stimulate [35S]guanosine-5′-O-(3-thio)triphosphate ([35S]GTPgammaS) binding and to inhibit adenylyl cyclase. These data show that endomorphins are capable of activating G proteins and inhibiting adenylyl cyclase activity, and all these effects are mediated by the mu opioid receptors.
Monory K, Bourin MC, Spetea M, et al. Specific activation of the mu opioid receptor (MOR) by endomorphin 1 and endomorphin 2. Eur J Neurosci. 2000;12(2):577-84.
Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex.
Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2) were previously isolated from bovine brain. Both peptides showed the greatest selectivity and affinity for the mu opiate receptor of any endogenous substance found to date and may serve as natural ligands for the mu-opiate receptor. We have purified them from the fronto-parietal cortex of human brain tissue by solid phase extraction and high performance liquid chromatography. Peptide content was followed by a specific and sensitive radioimmunoassay with an antibody that was generated against endomorphin-1. The isolated endomorphins showed full biological activity. The tetrapeptides were found in human brain in much higher amounts than in bovine frontal cortex.
Hackler L, Zadina JE, Ge LJ, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18(10):1635-9.
A potent and selective endogenous agonist for the mu-opiate receptor.
Peptides have been identified in mammalian brain that are considered to be endogenous agonists for the delta (enkephalins) and kappa (dynorphins) opiate receptors, but none has been found to have any preference for the mu receptor. Because morphine and other compounds that are clinically useful and open to abuse act primarily at the mu receptor, it could be important to identify endogenous peptides specific for this site. Here we report the discovery and isolation from brain of such a peptide, endomorphin-1 (Tyr-Pro-Trp-Phe-NH2), which has a high affinity (Ki = 360 pM) and selectivity (4,000- and 15,000-fold preference over the delta and kappa receptors) for the mu receptor. This peptide is more effective than the mu-selective analogue DAMGO in vitro and it produces potent and prolonged analgesia in mice. A second peptide, endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), which differs by one amino acid, was also isolated. The new peptides have the highest specificity and affinity for the mu receptor of any endogenous substance so far described and they may be natural ligands for this receptor.
Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386(6624):499-502.
Schematics