a Novel skeletal muscle-derived and Bone-specific Secreted Protein that Modulates the Osteoblast Phenotype
Abstract
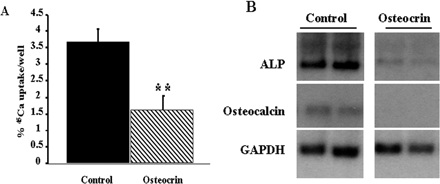
Osteocrin is a specific ligand of the natriuretic Peptide clearance receptor that modulates bone growth.
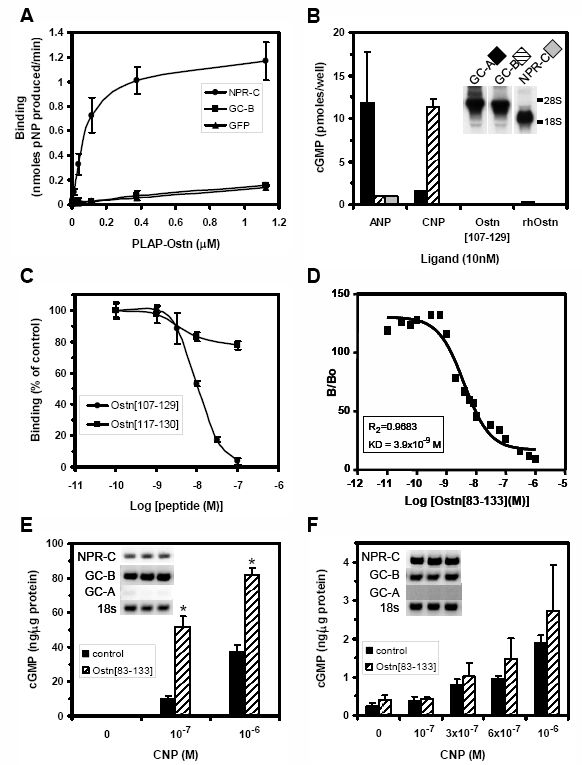
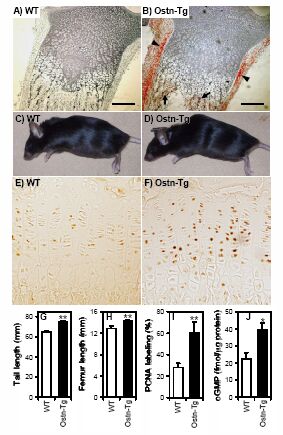
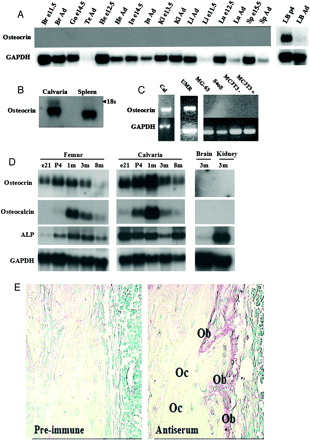
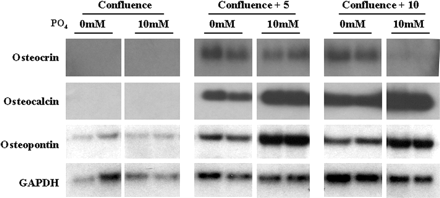
Osteocrin (Ostn) is a recently discovered secreted protein produced by cells of the osteoblast lineage that shows a well conserved homology with members of the natriuretic peptide (NP) family. We hypothesized that Ostn could interact with the NP receptors, thereby modulating NP actions on the skeleton. Ostn binds specifically and saturably to the NP peptide receptor-C (NPR-C) receptor with a Kd of approximately 5 nM with no binding to the GC-A or GC-B receptors. Deletion of several of the residues deemed important for NP binding to NPR-C led to abolition of Ostn binding, confirming the presence of a “natriuretic motif.” Functionally, Ostn was able to augment C-type natriuretic peptide-stimulated cGMP production in both pre-chondrocytic (ATDC5) and osteoblastic (UMR106) cells, suggesting increased NP levels due to attenuation of NPR-C associated NP clearance. Ostn-transgenic mice displayed elongated bones and a marked kyphosis associated with elevated bone cGMP levels, suggesting that elevated natriuretic peptide activity contributed to the increased bone length possibly through an increase in growth plate chondrocyte proliferation. Thus, we have demonstrated that Ostn is a naturally occurring ligand of the NPR-C clearance receptor and may act to locally modulate the actions of the natriuretic system in bone by blocking the clearance action of NPR-C, thus locally elevating levels of C-type natriuretic peptide.
Moffatt P, Thomas G, Sellin K, et al. Osteocrin is a specific ligand of the natriuretic Peptide clearance receptor that modulates bone growth. J Biol Chem. 2007;282(50):36454-62.
Musclin, a novel skeletal muscle-derived secretory factor.
Skeletal muscle is involved in the homeostasis of glucose and lipid metabolism. We hypothesized that the skeletal muscle produces and secretes bioactive factor(s), similar to adipocytokines secreted by fat tissue. Here, we report the identification of a novel secretory factor, musclin, by signal sequence trap (SST) of mouse skeletal muscle cDNAs. Musclin cDNA encoded 130 amino acids, including N-terminal 30-amino acid signal sequence. Musclin protein contained a region homologous to natriuretic peptide (NP) family, and KKKR, a putative serine protease cleavage site, similar to NP family. Full-length musclin protein and KKKR-dependent cleaved form were secreted in media of musclin cDNA-transfected mammalian cell cultures. Musclin mRNA was expressed almost exclusively in the skeletal muscle of mice and rats. Musclin mRNA levels in skeletal muscle were markedly low in fasted, increased upon re-feeding, and were low in streptozotocin-treated insulin-deficient mice. Musclin mRNA expression was induced at late stage in the differentiation of C2C12 myocytes. In myocytes, insulin increased while epinephrine, isoproterenol and forskolin reduced musclin mRNA, all of which are known to increase the cellular content of cyclic AMP, a counter regulator to insulin. Pathologically, overexpression of musclin mRNA was noted in the muscles of obese insulin-resistant KKAy and db/db mice. Functionally, recombinant musclin significantly attenuated insulin-stimulated glucose uptake in myocytes. In conclusion, we identified musclin, a novel skeletal muscle-derived secretory factor. Musclin expression level is tightly regulated by nutritional changes and its physiological role could be linked to glucose metabolism.
Nishizawa H, Matsuda M, Yamada Y, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279(19):19391-5.
Schematics


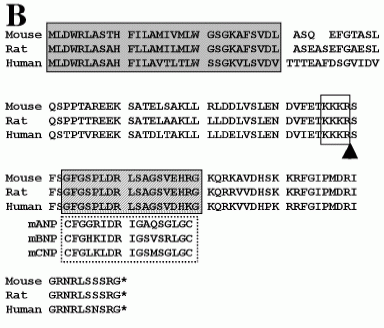 |
Alignments of musclin amino acid sequences for mouse, rat and human. Predicted signal sequence; shaded box. The putative serine protease cleavage site, KKKR; open box. The region homologous to mouse ANP, BNP and CNP; hatched box. |
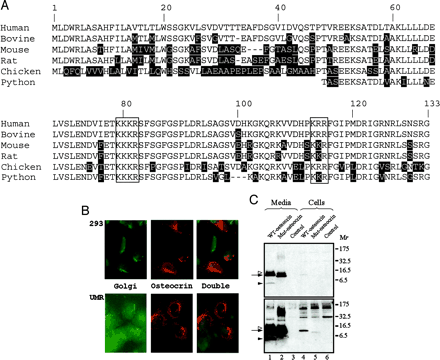
A, predicted amino acid sequences from human, bovine, mouse, rat, chicken, and snake (partial) cDNAs. The two dibasic cleavage sites, 76KKKR79 and 110KKR112, are well conserved (boxed). Shaded residues represent non-conserved residues from the human sequence. Note that the C-terminal half of the protein is highly conserved.B, immunofluorescent localization with a human osteocrin-specific antibody demonstrates overexpressed osteocrin is clearly visible in the secretory apparatus of HEK293A fibroblasts and UMR106 osteosarcoma cells (x400) colocalizing with the 58K Golgi protein-specific antibody.C, the majority of osteocrin is detected in the medium of HEK293 cells by Western blot when transiently transfected (lanes 1 and 2). Residual protein in the secretory apparatus is detected in the cell lysate (lanes 4 and 5). Two exposures of the Western blot are shown to illustrate the underrepresented smaller processed fragments, with the lower panel representing a longer exposure. A doublet can be seen at the expected full-length size of 11.4 kDa and smaller processed fragments at ~ 5 kDa (lane 1). Mutation of the KKKR site to AS abolishes processing of the protein leaving only full-length 11.4 kDa bands (lane 2). Equal proportions of media or cell lysate from cultured cells were loaded. WT, wild type; Mut, mutated.
More Information