C-Terminal Prothrombin, TCP-25: A dual-action anti-inflammatory/antibacterial peptide
Abstract
A dual-action peptide-containing hydrogel targets wound infection and inflammation.
Puthia M, Butrym M, Petrlova J, et al. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci Transl Med. 2020;12(524)
Thrombin-Derived Host-Defense Peptides Modulate Monocyte/Macrophage Inflammatory Responses to Gram-Negative Bacteria.
Abstract: Host-defense peptides play a fundamental role in the innate immune system by modulating inflammatory responses. Previously, it was shown that the thrombin derived host-defense peptide GKY25 inhibits LPS-induced responses of monocytes and macrophages in vitro, ex vivo, and in vivo. In this study, the effect of GKY25 on the interaction of monocytes/macrophages with Gram-negative bacteria was explored. Electron microscopy analysis showed that fibrin slough from non-healing wounds, colonized with Staphylococcus aureus and Pseudomonas aeruginosa, contains C-terminal thrombin epitopes associated with these bacteria extracellularly and in phagosomes of leukocytes. Live imaging of RAW 264.7 cell cultures showed binding of GKY25 to Escherichia coli BioParticles extracellularly, and colocalization intracellularly. Although peptide binding did not alter the rate of phagocytosis, GKY25 reduced NF-κB/AP-1 activation and subsequent cytokine release in response to both heat-killed and live bacteria. Notably, preincubation of RAW 264.7 cells with peptide did increase BioParticle uptake in a dose-dependent manner. Taken together, the thrombin-derived host-defense peptide GKY25 binds to bacteria extracellularly and colocalizes with bacteria intracellularly, thereby reducing pro-inflammatory responses.
Hansen FC, Strömdahl AC, Mörgelin M, Schmidtchen A, Van der plas MJA. Thrombin-Derived Host-Defense Peptides Modulate Monocyte/Macrophage Inflammatory Responses to Gram-Negative Bacteria. Front Immunol. 2017;8:843.
Thrombin-derived host defence peptide modulates neutrophil rolling and migration in vitro and functional response in vivo.
Abstract: Host defence peptides (HDPs) derived from the C-terminus of thrombin are proteolytically generated by enzymes released during inflammation and wounding. In this work, we studied the effects of the prototypic peptide GKY25 (GKYGFYTHVFRLKKWIQKVIDQFGE), on neutrophil functions. In vitro, GKY25 was shown to decrease LPS-induced neutrophil activation. In addition, the peptide induced CD62L shedding on neutrophils without inducing their activation. Correspondingly, GKY25-treated neutrophils showed reduced attachment and rolling behaviour on surfaces coated with the CD62L ligand E-selectin. The GKY25-treated neutrophils also displayed a dampened chemotactic response against the chemokine IL-8. Furthermore, in vivo, mice treated with GKY25 exhibited a reduced local ROS response against LPS. Taken together, our results show that GKY25 can modulate neutrophil functions in vitro and in vivo.
Lim CH, Puthia M, Butrym M, et al. Thrombin-derived host defence peptide modulates neutrophil rolling and migration in vitro and functional response in vivo. Sci Rep. 2017;7(1):11201.
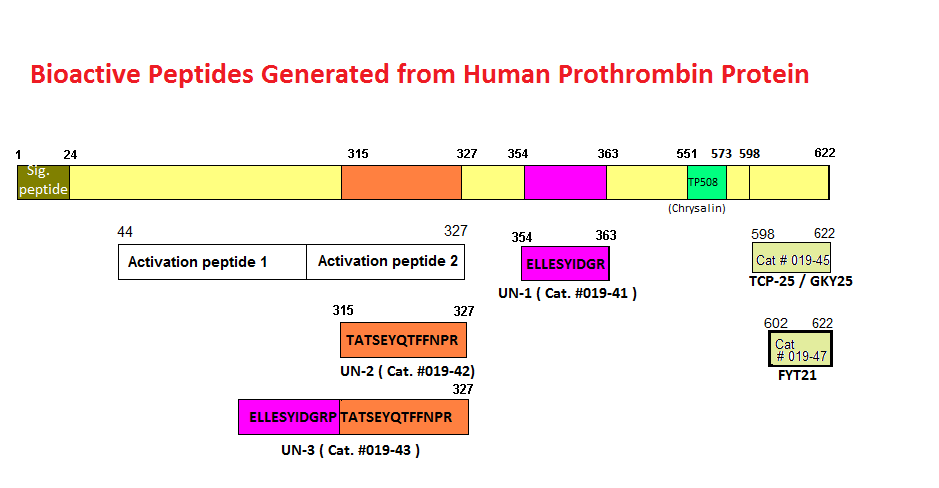
Schematics