A multifunctional cytokine with effects on lipid metabolism, coagulation, insulin resistance, and endothelial function
Schematics
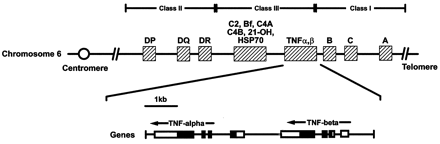
Structure of the TNF genes. The locations of MHC loci are shown. Hatched boxes indicate the relative positions of the indicated loci. Open boxes represent the untranslated portions of the TNF exons, and closed boxes represent translated portions. Lines indicate introns (areas of the gene that are spliced out of the mature RNA product). Arrows indicate the transcriptional orientation of the genes. Reprinted with permission by Webb et al.26 kb = kilobase. C2 = complement component 2; Bf = complement factor B; C4A = complement component 4A; C4B = complement component C4B; 21-OH = 21-hydroxylase.

More Information

Metabolism and immunity are closely linked. Both overnutrition and undernutrition have implications for immune function. Starvation and malnutrition can suppress immune function and increase susceptibility to infections. Obesity is associated with a state of aberrant immune activity and increasing risk for associated inflammatory diseases, including atherosclerosis, diabetes, airway inflammation, and fatty liver disease. Thus, optimal nutritional and metabolic homeostasis is an important part of appropriate immune function and good health.
Kathryn E. Wellen and Gökhan S. Hotamisligil. J. Clin. Invest. 115:1111-1119 (2005)
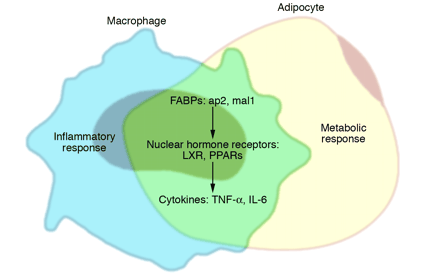
Lipids and inflammatory mediators: integration of metabolic and immune responses in adipocytes and macrophages through shared mechanisms. Under normal conditions, adipocytes store lipids and regulate metabolic homeostasis, and macrophages function in the inflammatory response, although each cell type has the capacity to perform both functions. In obesity, adipose tissue becomes inflamed, both via infiltration of adipose tissue by macrophages and as a result of adipocytes themselves becoming producers of inflammatory cytokines. Inflammation of adipose tissue is a crucial step in the development of peripheral insulin resistance. In addition, in proatherosclerotic conditions such as obesity and dyslipidemia, macrophages accumulate lipid to become foam cells. Adipocytes and macrophages share common features such as expression of cytokines, FABPs, nuclear hormone receptors, and many other factors. As evidenced by genetic loss-of-function models, adipocyte/macrophage FABPs modulate both lipid accumulation in adipocytes and cholesterol accumulation in macrophages, as well as the development of insulin resistance and atherosclerosis. PPAR and LXR pathways oppose inflammation and promote cholesterol efflux from macrophages and lipid storage in adipocytes.
Kathryn E. Wellen and Gökhan S. Hotamisligil. J. Clin. Invest. 115:1111-1119 (2005)
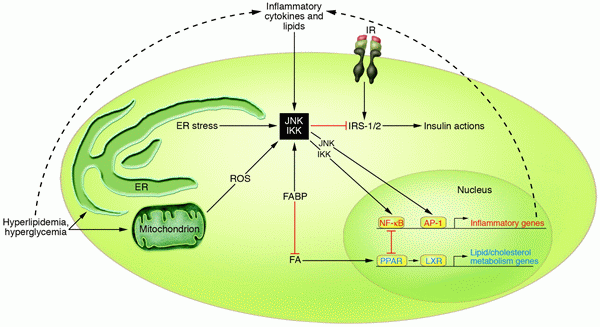
Model of overlapping metabolic and inflammatory signaling and sensing pathways in adipocytes or macrophages. Inflammatory pathways can be initiated by extracellular mediators such as cytokines and lipids or by intracellular stresses such as ER stress or excess ROS production by mitochondria. Signals from all of these mediators converge on inflammatory signaling pathways, including the kinases JNK and IKK. These pathways lead to the production of additional inflammatory mediators through transcriptional regulation as well as to the direct inhibition of insulin signaling. Other pathways such as those mediated through the SOCS proteins and iNOS are also involved in inflammation-mediated inhibition of insulin action. Opposing the inflammatory pathways are transcription factors from the PPAR and LXR families, which promote nutrient transport and metabolism and antagonize inflammatory activity. More proximal regulation is provided by FABPs, which likely sequester ligands of these transcription factors, thus promoting a more inflammatory environment. The absence of FABPs is antiinflammatory. The cell must strike a balance between metabolism and inflammation. In conditions of overnutrition, this becomes a particular challenge, as the very processes required for response to nutrients and nutrient utilization, such as mitochondrial oxidative metabolism and increasing protein synthesis in the ER, can induce the inflammatory response. IR, insulin receptor.
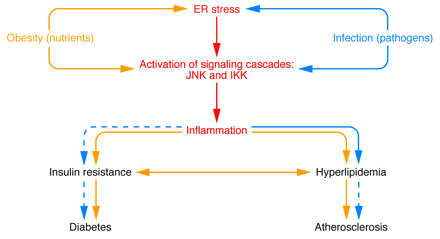
Nutrient and pathogen sensing or response systems have important overlapping features, and their modulation by obesity or infection can lead to overlapping physiological outcomes. For example, the chronic inflammation of obesity leads to elevated plasma lipid levels and the development of insulin resistance, eventually resulting in fatty liver disease, atherosclerosis, and diabetes. Infection typically leads to a more transient and robust inflammatory response and short-term hyperlipidemia that aids in the resolution of the infection. In some circumstances of chronic infection, however, insulin resistance, diabetes, and atherosclerosis can result.
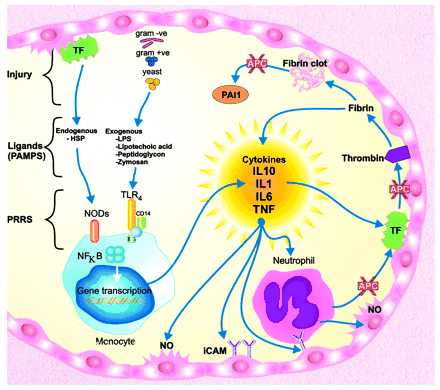
A simplified diagram of the innate immune response to infection and tissue injury involving the inflammatory cytokines and the coagulation cascade. –ve = negative; +ve = positive; PAI1 = platelet-activation inhibitor-1; ICAM = intracellular adhesion molecule.

Effect of salicylates on insulin signaling during insulin resistance. (a) Normally, the occupied insulin receptor phosphorylates scaffold proteins, such as the IRS-1, on critical tyrosine residues. However, in insulin-resistant states, a number of agents, such as the cytokine TNF- or circulating FFAs, lead through intermediary signaling pathways to the activation of IKK, which in turn indirectly increases the number of phosphorylated serine and threonine residues (indicated by blue circles) on IRS-1. This modification blocks the tyrosine phosphorylation and converts IRS-1 into an insulin receptor inhibitory protein. (b) In the presence of salicylates, IKK activity is inhibited, reducing the IRS-1 serine/threonine phosphorylation and allowing IRS-1 to be phosphorylated on tyrosine. These phosphorylated tyrosine residues (black squares) serve as binding sites for a number of signaling molecules, most importantly PI 3′-kinase, which initiate signaling pathways regulating metabolism. Aspirin (ASA) also inhibits cyclooxygensases (COX) to prevent the generation of inflammatory prostaglandins (PGE) from arachidonic acid (AA) in a pathway unrelated to the effects of the drug on insulin action.
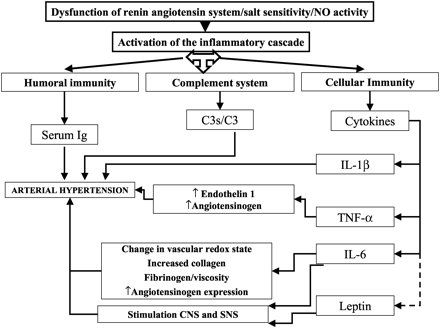
Proposed pathophysiology of hypertension through inflammatory mechanisms (see Section II). CNS, Central nervous system; SNS, sympathetic nervous system. José Manuel Fernández-Real and Wifredo Ricart . Endocrine Reviews 24 (3): 278-301
