TREM2 and TREML2 are drug targets to attenuate Alzheimer's disease
Abstract
Peripheral sTREM2-Related Inflammatory Activity Alterations in Early-Stage Alzheimer’s Disease
Abstract: Alzheimer’s disease (AD) has been linked to multiple immune system-related genetic variants. Triggering receptor expressed on myeloid cells 2 (TREM2) genetic variants are risk factors for AD and other neurodegenerative diseases. In addition, soluble TREM2 (sTREM2) isoform is elevated in cerebrospinal fluid in the early stages of AD and is associated with slower cognitive decline in a disease stage-dependent manner. Multiple studies have reported an altered peripheral immune response in AD. However, less is known about the relationship between peripheral sTREM2 and an altered peripheral immune response in AD. The objective of this study was to explore the relationship between human plasma sTREM2 and inflammatory activity in AD. The hypothesis of this exploratory study was that sTREM2-related inflammatory activity differs by AD stage. We observed different patterns of inflammatory activity across AD stages that implicate early-stage alterations in peripheral sTREM2-related inflammatory activity in AD. Notably, fractalkine showed a significant relationship with sTREM2 across different analyses in the control groups that was lost in later AD-related stages with high levels in mild cognitive impairment. Although multiple other inflammatory factors either differed significantly between groups or were significantly correlated with sTREM2 within specific groups, three inflammatory factors (fibroblast growth factor-2, GM-CSF, and IL-1β) are notable because they exhibited both lower levels in AD, compared with mild cognitive impairment, and a change in the relationship with sTREM2. This evidence provides important support to the hypothesis that sTREM2-related inflammatory activity alterations are AD stage specific and provides critical information for therapeutic strategies focused on the immune response.
Weber GE, Khrestian M, Tuason ED, et al. Peripheral strem2-related inflammatory activity alterations in early-stage alzheimer’s disease. JI. 2022;208(10):2283-2299.
Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: a longitudinal observational study
Background: Therapeutic modulation of TREM2-dependent microglial function might provide an additional strategy to slow the progression of Alzheimer’s disease. Although studies in animal models suggest that TREM2 is protective against Alzheimer’s pathology, its effect on tau pathology and its potential beneficial role in people with Alzheimer’s disease is still unclear. Our aim was to study associations between the dynamics of soluble TREM2, as a biomarker of TREM2 signalling, and amyloid β (Aβ) deposition, tau-related pathology, neuroimaging markers, and cognitive decline, during the progression of autosomal dominant Alzheimer’s disease.
Morenas-Rodríguez E, Li Y, Nuscher B, et al. Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: a longitudinal observational study. The Lancet Neurology. 2022;21(4):329-341.
Soluble TREM2: Innocent bystander or active player in neurological diseases?
Abstract: Triggering receptor expressed on myeloid cells 2 (TREM2) is an innate immune receptor expressed by macrophages and microglia in the central nervous system (CNS). TREM2 has attracted a lot of interest in the past decade for its critical role in modulating microglia functions under homeostatic conditions and in neurodegenerative diseases. Genetic variation in TREM2 is sufficient to cause Nasu-Hakola disease, a rare pre-senile dementia with bone cysts, and to increase risk for Alzheimer’s disease, frontotemporal dementia, and other neurodegenerative disorders. Beyond the role played by TREM2 genetic variants in these diseases, TREM2 engagement is a key step in microglia activation in response to different types of tissue injury (e.g. β-Amyloid deposition, demyelination, apoptotic cell death) leading to enhanced microglia metabolism, phagocytosis, proliferation and survival. TREM2 also exists as a soluble form (sTREM2), generated from receptor shedding or alternative splicing, which is detectable in plasma and cerebrospinal fluid (CSF). Genetic variation, physiological conditions and disease status impact CSF sTREM2 levels. Clinical and preclinical studies suggest that targeting and/or monitoring sTREM2 could have clinical and therapeutic implications. Despite the critical role of sTREM2 in neurologic disease, its function remains poorly understood. Here, we review the current literature on sTREM2 regarding its origin, genetic variation, and possible functions as a biomarker in neurological disorders and as a potential active player in CNS diseases and target for therapies.
Filipello F, Goldsbury C, You SF, Locca A, Karch CM, Piccio L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiology of Disease. 2022;165:105630.
The Alzheimer’s disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation
Wang SY, Fu XX, Duan R, et al. The Alzheimer’s disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation. Neural Regen Res. 2023;18(2):434.
Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading
Jain N, Lewis CA, Ulrich JD, Holtzman DM. Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading. Journal of Experimental Medicine. 2023;220(1):e20220654.
Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation
Zheng H, Liu CC, Atagi Y, et al. Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiology of Aging. 2016;42:132-141.
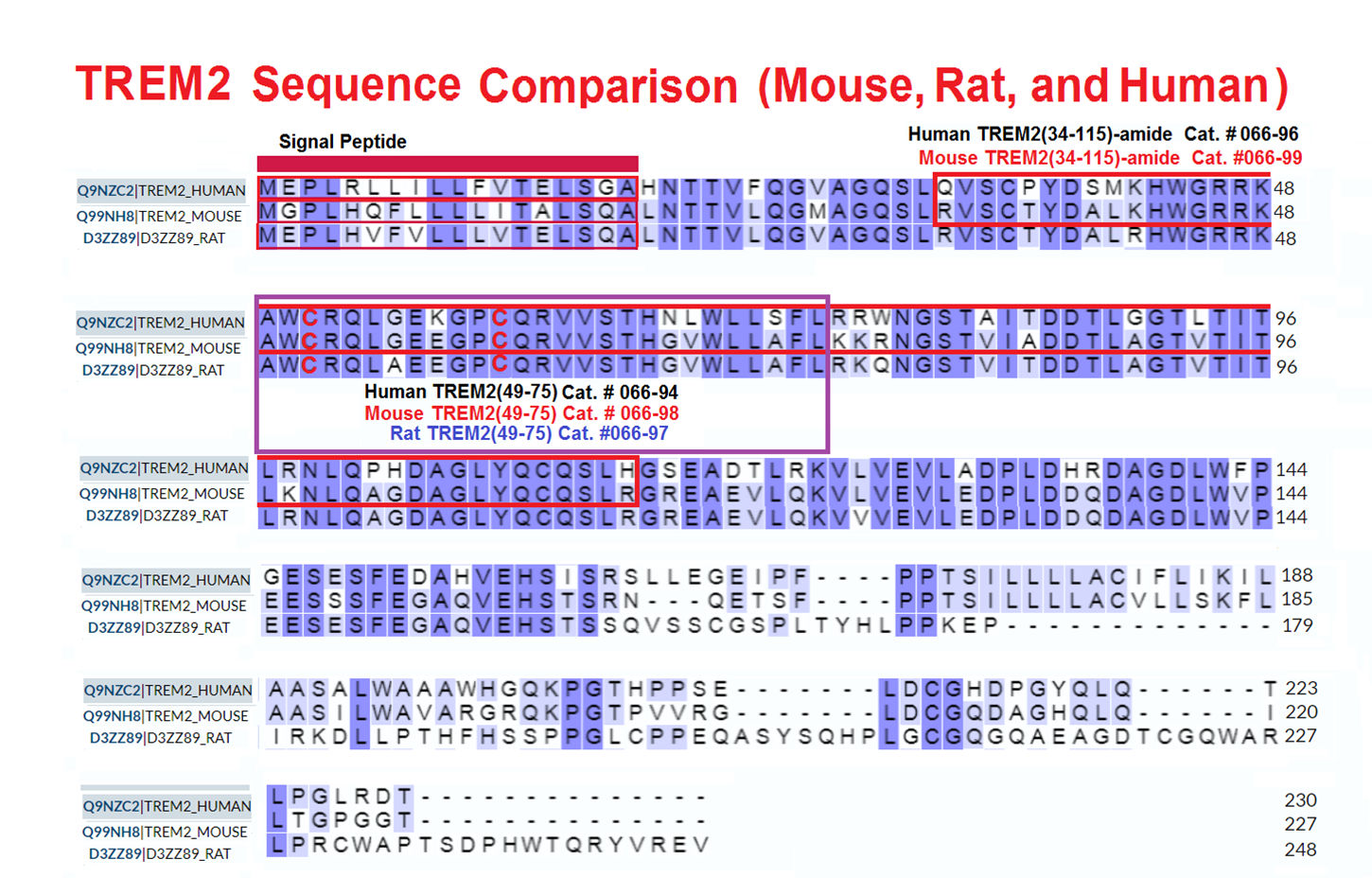
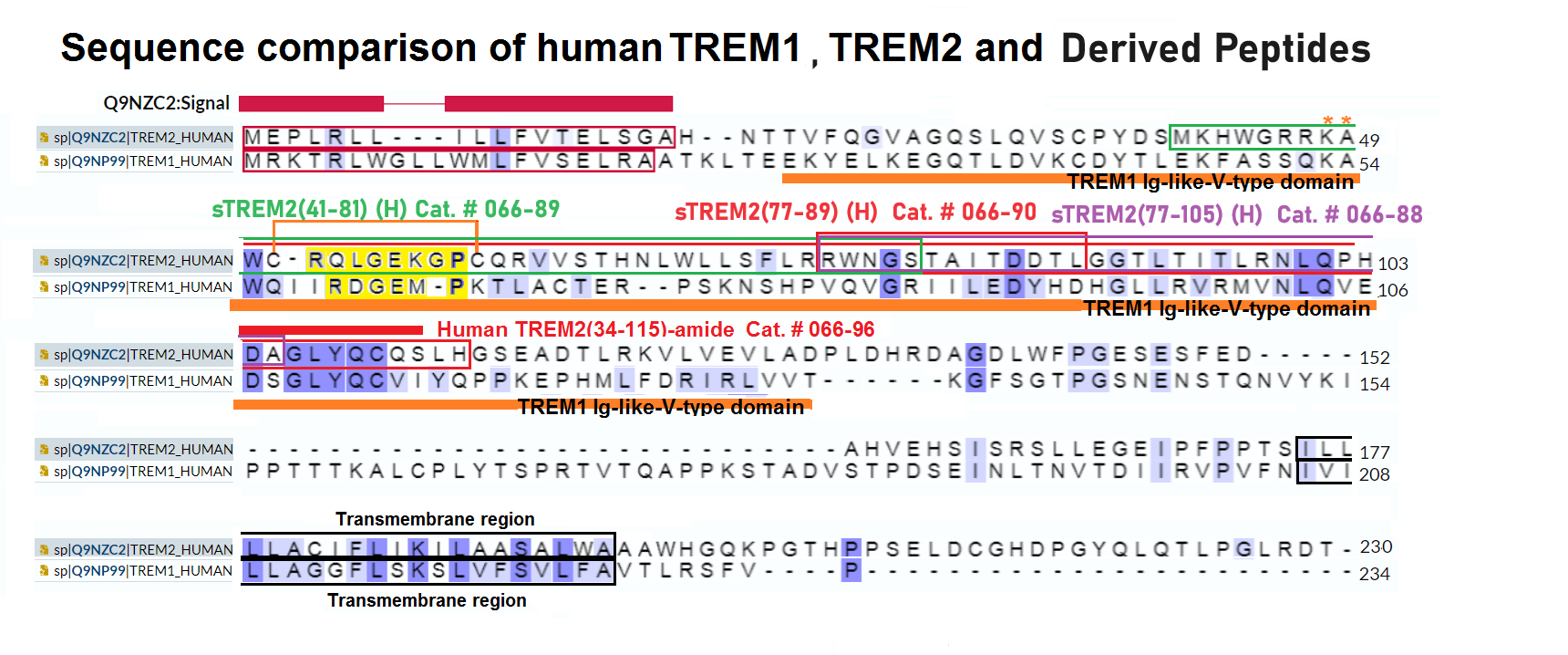
Schematics